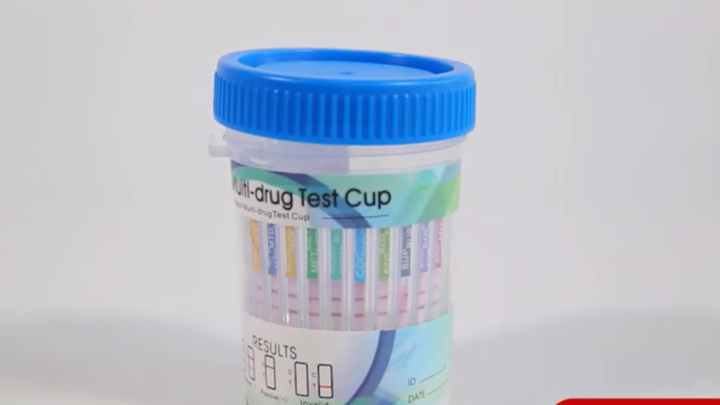
Medical Diagnostics & Screening 12 panel urine testing cup clia-waived
DOA drug of abuse diagnostics test kits Format: Strip, Cassette, Dipcard, Cup
Rapid Single/Multi-drug Test Dipcard/Cup is a rapid, screening test for the qualitative detection of single/multiple drugs and drug metabolites in human urine at specified cut off levels.
Rapid Single/Multi-drug Test Dipcard is an immuno-chromatographic assay for the qualitative determination of the presence of drugs listed in the table below.
Instruction of use for testing of any combination of the following drugs:
AMP/ BAR/ BUP / BZO/ COC/ COT/ ETG/ FYL/ K2/ KET/ MAM/ MDMA
MET/ MOP300/ MTD/ OPI2000/ OXY/ PCP/ PPX/ TCA/ THC/ TRA
For professional use only. For in vitro diagnostic use only.
INTENDED USE
| Drug Test (Identifier) | Cut-off level(ng/mL) |
| Rapid Amphetamine (AMP) | 1000ng/ml |
| Rapid Barbiturates (BAR) | 300ng/ml |
| Rapid Benzodiazepines (BZO) | 300ng/ml |
| Rapid Buprenorphine (BUP) | 10ng/ml |
| Rapid Cocaine (COC) | 300ng/ml |
| Rapid Marijuana (THC) | 50ng/ml |
| Rapid Methamphetamine (MET) | 1000ng/ml |
| Rapid Methadone (MTD) | 300ng/ml |
| Rapid Methadone Metabolite (EDDP) | 300ng/ml |
| Methylenedioxymethamphetamine-Ecstasy (MDMA) | 500ng/ml |
| Morphine 300 (MOP) | 300ng/ml |
| Rapid Opiate 2000 (OPI) | 2000ng/ml |
| Rapid Oxycodone (OXY) | 100ng/ml |
| Rapid Phencyclidine (PCP) | 25ng/ml |
| Rapid Propoxyphene (PPX) | 300ng/ml |
| Rapid Tri-cyclic Antidepressants (TCA) Test | 1000ng/ml |
| Rapid Cotinine (COT) | 200ng/ml |
| Rapid Ethyl Glucuronide (ETG) | 500ng/ml |
| Synthetic Marijuana (K2) | 50ng/ml |
| Rapid Fentanyl (FYL) | 200ng/ml |
| Ketamine(KET) | 1000ng/ml |
| 6-Monoacetylmorphine (MAM) | 10ng/mL |
| Tramadol (TRA) | 200 ng/mL |
Product Shows:

How to test:

Product Formats:
1, Strip 2, dipcard 3, cassette 4, multi-drug test cup

![]()
![]() Factory Pictures:
Factory Pictures:
Co-Innovation is an innovative and creative enterprise in R&D, manufacturing and marketing of rapid diagnostics tests. In 2012, Co-innovation was established in Guangzhou, China. Specialized in lateral flow immunoassay technology, we provide diagnostics tests to global market with pregnancy tests, ovulation predictor, drug of abuse tests. Our products are being sold to many countries, including United States, Germany, Netherlands, England, Chile, Ecuador, Malaysia, Singapore, India, Australia etc. Our products have been widely recognized and enormously supported, we aim to produce high cost-effective products, and create the greatest value for customers.

ISO 13485, USA 510k, CE, Clia-waived

Application:

Buyer Feedback:

| Contact us |
Contact Person: Emily Li
Cel: +86-15920342793
Tel: 020-82109823
Skype: emilylee20091 Wechat/WhatsApp: +86-15920342793
Add: No. 9 Baihe 3 Street, Economic And Technological Development East Zone, Guangzhou 510530, Guangdong P.R. China.













Reviews
There are no reviews yet.