-
Colgate Extra Clean Single Toothbrush ₦3,900.00 QTY: 1
-
Superdren Thermo 60cps ₦37,924.00 QTY: 1
-
CLOPITAB CV 20MG CAP 15`S ₦8,711.75 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
103249–103264 of 356215 Results
-
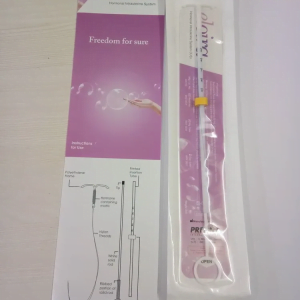
Eloira Hormonel Intrauterine System IusSku: #ME01642853
Eloira Hormonel Intrauterine System Ius
₦22,800.00 -
ELON MUSK: Tesla, SpaceX and the quest for a fantastic future Weight 0.5 kg Author Ashlee Vance Publisher Virgin Books Pages Paperback (402)Sku: 1691056054-100
ELON MUSK: Tesla, SpaceX and the quest for a fantastic future
₦6,000.00 -
-
-
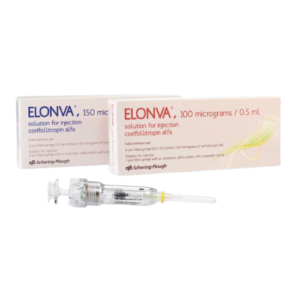
Controlled Ovarian Stimulation (COS) in combination with a GnRH antagonist for the development of multiple follicles in women participating in an Assisted Reproductive Technology (ART) program.Sku: 1727472299-12
Elonva?
₦1,728,000.00 -
SaleElopag 25 mgElopag 25 mg is indicated in Chronic Immune (Idiopathic) Thrombocytopenia, Chronic Hepatitis C-associated Thrombocytopenia, Severe Aplastic Anemia.Theropeutic ClassHaemostatic drugsPharmacologyEltrombopag is an orally bioavailable, small-molecule TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor. Eltrombopag is a stimulator of STAT and JAK phosphorylation. Unlike recombinant TPO or romiplostim, Eltrombopag does not activate the AKT pathway in any way. It should be noted that when given to patients with aplastic anemia, other lineages besides platelet count were increased, suggesting that either eltrombopag enhanced the effect of TPO in vivo; or there is a yet uncovered mechanism of action at work.Dosage & Administration of Elopag 25 mgChronic Immune (Idiopathic) Thrombocytopenia- Adult and Pediatric Patients 6 Years and Older with ITP: Initiate Eltrombopag at a dose of 50 mg once daily, except in patients who are of East Asian ancestry (such as Chinese, Japanese, Taiwanese or Korean) or who have mild to severe hepatic impairment (Child-Pugh Class A,B,C). For patients of East Asian ancestry with ITP, initiate Eltrombopag at a reduced dose of 25 mg once daily. For patients with ITP and mild, moderate or severe hepatic impairment (Child-Pugh Class A,B,C), initiate Eltrombopag at a reduced dose of 25 mg once daily. For patients of East Asian ancestry with ITP and hepatic impairment (Child-Pugh Class A,B,C), consider initiating Eltrombopag at a reduced dose of 12.5 mg once daily. Pediatric Patients with ITP Aged 1 to 5 Years: Initiate Eltrombopag at a dose of 25 mg once daily. Chronic Hepatitis C-associated Thrombocytopenia- Initiate Eltrombopag at a dose of 25 mg once daily. Monitoring and Dose Adjustment: Adjust the dose of Eltrombopag in 25-mg increments every 2 weeks as necessary to achieve the target platelet count required to initiate antiviral therapy. Monitor platelet counts every week prior to starting antiviral therapy. During antiviral therapy, adjust the dose of Eltrombopag to avoid dose reductions of Peginterferon. Monitor CBCs with differentials, including platelet counts, weekly during antiviral therapy until a stable platelet count is achieved. Monitor platelet counts monthly thereafter. Do not exceed a dose of 100 mg daily. Monitor clinical hematology and liver tests regularly throughout therapy with Eltrombopag. Severe Aplastic Anemia- Initiate Eltrombopag at a dose of 50 mg once daily. For patients with severe aplastic anemia of East Asian ancestry or those with mild, moderate or severe hepatic impairment (Child-Pugh Class A,B,C), initiate Eltrombopag at a reduced dose of 25 mg once daily. Dosage of Elopag 25 mgChronic Immune (Idiopathic) Thrombocytopenia- Adult and Pediatric Patients 6 Years and Older with ITP: Initiate Eltrombopag at a dose of 50 mg once daily, except in patients who are of East Asian ancestry (such as Chinese, Japanese, Taiwanese or Korean) or who have mild to severe hepatic impairment (Child-Pugh Class A,B,C). For patients of East Asian ancestry with ITP, initiate Eltrombopag at a reduced dose of 25 mg once daily. For patients with ITP and mild, moderate or severe hepatic impairment (Child-Pugh Class A,B,C), initiate Eltrombopag at a reduced dose of 25 mg once daily. For patients of East Asian ancestry with ITP and hepatic impairment (Child-Pugh Class A,B,C), consider initiating Eltrombopag at a reduced dose of 12.5 mg once daily. Pediatric Patients with ITP Aged 1 to 5 Years: Initiate Eltrombopag at a dose of 25 mg once daily. Chronic Hepatitis C-associated Thrombocytopenia- Initiate Eltrombopag at a dose of 25 mg once daily. Monitoring and Dose Adjustment: Adjust the dose of Eltrombopag in 25-mg increments every 2 weeks as necessary to achieve the target platelet count required to initiate antiviral therapy. Monitor platelet counts every week prior to starting antiviral therapy. During antiviral therapy, adjust the dose of Eltrombopag to avoid dose reductions of Peginterferon. Monitor CBCs with differentials, including platelet counts, weekly during antiviral therapy until a stable platelet count is achieved. Monitor platelet counts monthly thereafter. Do not exceed a dose of 100 mg daily. Monitor clinical hematology and liver tests regularly throughout therapy with Eltrombopag. Severe Aplastic Anemia- Initiate Eltrombopag at a dose of 50 mg once daily. For patients with severe aplastic anemia of East Asian ancestry or those with mild, moderate or severe hepatic impairment (Child-Pugh Class A,B,C), initiate Eltrombopag at a reduced dose of 25 mg once daily. Interaction of Elopag 25 mgTake Eltrombopag at least 2 hours before or 4 hours after any medications or products containing polyvalent cations such as antacids, calcium-rich foods and mineral supplements.ContraindicationsThere are no contraindications for EltrombopagSide Effects of Elopag 25 mgThe most common side effects of Eltrombopag in adults when used to treat chronic ITP are: In adult patients with ITP, the most common adverse reactions (greater than or equal to 5% and greater than placebo) were: nausea, diarrhea, upper respiratory tract infection, vomiting, increased ALT, myalgia and urinary tract infection. In pediatric patients age 1 year and older with ITP, the most common adverse reactions (greater than or equal to 10% and greater than placebo) were upper respiratory tract infection and nasopharyngitis.In patients with chronic hepatitis C-associated thrombocytopenia, the most common adverse reactions (greater than or equal to 10% and greater than placebo) were: anemia, pyrexia, fatigue, headache, nausea, diarrhea, decreased appetite, influenza-like illness, asthenia, insomnia, cough, pruritus, chills, myalgia, alopecia and peripheral edema. In patients with severe aplastic anemia, the most common adverse reactions (greater than or equal to 20%) were: nausea, fatigue, cough, diarrhea and headache.Pregnancy & LactationPregnancy Category C. There are no adequate and well controlled studies of Eltrombopag usein pregnancy. In animal reproduction and developmental toxicity studies, there was evidence ofembryolethality and reduced fetal weights at maternally toxic doses. Eltrombopag should beused in pregnancy only if the potential benefit to the mother justifies the potential risk to thefetus.Nursing Mothers: It is not known whether Eltrombopag is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Eltrombopag, a decision should be made whether to discontinue nursing or to discontinue Eltrombopag taking into account the importance of Eltrombopag to the mother.Precautions & WarningsHepatotoxicity: Monitor liver function before and during therapy. Thrombotic or Thromboembolic Complications: Portal vein thrombosis has been reported in patients with chronic liver disease receiving Eltrombopag. Monitor platelet counts regularly.Overdose Effects of Elopag 25 mgIn the event of overdose, platelet counts may increase excessively and result in thrombotic/thromboembolic complications.In one report, a subject who ingested 5,000 mg of Eltrombopag had a platelet count increase to a maximum of 929 x 109/L at 13 days following the ingestion. The patient also experienced rash, bradycardia, ALT/AST elevations and fatigue. The patient was treated with gastric lavage, oral Lactulose, Intravenous fluids, Omeprazole, Atropine, Furosemide, Calcium, Dexamethasone, andPlasmapheresis; however, the abnormal platelet count and liver test abnormalities persisted for 3 weeks. After 2 months follow-up, all events had resolved without sequelae.In case of an overdose, consider oral administration of a metal cation-containing preparation, such as Calcium, Aluminum, or Magnesium preparations to chelate Eltrombopag and thus limit absorption. Closely monitor platelet counts. Reinitiate treatment with Eltrombopag in accordance with dosing and administration recommendations.Storage ConditionsStore in a cool and dry place, away from light and moisture. Keep out of the reach of children.Use In Special PopulationsPediatric Use:The safety and efficacy of Eltrombopag in pediatric patients 1 year and older with chronic ITP were evaluated in two double-blind, placebo-controlled. The pharmacokinetics of Eltrombopag has been evaluated in 168 pediatric patients 1 year and older with ITP dosed once daily for dosing recommendations for pediatric patients 1 year and older. The safety and efficacy ofEltrombopag in pediatric patients younger than 1 year with ITP have not yet been established. The safety and efficacy of Eltrombopag in pediatric patients with thrombocytopenia associated with chronic hepatitis C and severe aplastic anemia have not been established.Geriatric Use: Of the 106 patients in two randomized clinical trials of Eltrombopag 50 mg in chronic ITP, 22% were 65 years of age and over, while 9% were 75 years of age and over. In the two randomized clinical trials of Eltrombopag in patients with chronic hepatitis C and thrombocytopenia, 7% were 65 years of age and over, while fewer than 1% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients in the placebo-controlled trials, but greater sensitivity of some older individuals cannot be ruled out.Hepatic Impairment: Hepatic impairment influences the exposure of Eltrombopag. Reduce the initial dose of Eltrombopag in patients with chronic ITP (adult and pediatric patients 6 years and older only) or severe aplastic anemia who also have hepatic impairment (Child-Pugh Class A, B, C). No dosage adjustment is necessary for patients with chronic hepatitis C and hepatic impairment.Renal Impairment: No adjustment in the initial dose of Eltrombopag is needed for patients with renal impairment. Closely monitor patients with impaired renal function when administering Eltrombopag.Drug ClassesHaemostatic drugsMode Of ActionEltrombopag is an orally bioavailable, small-molecule TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor. Eltrombopag is a stimulator of STAT and JAK phosphorylation. Unlike recombinant TPO or romiplostim, Eltrombopag does not activate the AKT pathway in any way. It should be noted that when given to patients with aplastic anemia, other lineages besides platelet count were increased, suggesting that either eltrombopag enhanced the effect of TPO in vivo; or there is a yet uncovered mechanism of action at work.PregnancyPregnancy Category C. There are no adequate and well controlled studies of Eltrombopag usein pregnancy. In animal reproduction and developmental toxicity studies, there was evidence ofembryolethality and reduced fetal weights at maternally toxic doses. Eltrombopag should beused in pregnancy only if the potential benefit to the mother justifies the potential risk to thefetus.Nursing Mothers: It is not known whether Eltrombopag is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Eltrombopag, a decision should be made whether to discontinue nursing or to discontinue Eltrombopag taking into account the importance of Eltrombopag to the mother.Pediatric UsesPediatric Use:The safety and efficacy of Eltrombopag in pediatric patients 1 year and older with chronic ITP were evaluated in two double-blind, placebo-controlled. The pharmacokinetics of Eltrombopag has been evaluated in 168 pediatric patients 1 year and older with ITP dosed once daily for dosing recommendations for pediatric patients 1 year and older. The safety and efficacy ofEltrombopag in pediatric patients younger than 1 year with ITP have not yet been established. The safety and efficacy of Eltrombopag in pediatric patients with thrombocytopenia associated with chronic hepatitis C and severe aplastic anemia have not been established.Geriatric Use: Of the 106 patients in two randomized clinical trials of Eltrombopag 50 mg in chronic ITP, 22% were 65 years of age and over, while 9% were 75 years of age and over. In the two randomized clinical trials of Eltrombopag in patients with chronic hepatitis C and thrombocytopenia, 7% were 65 years of age and over, while fewer than 1% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients in the placebo-controlled trials, but greater sensitivity of some older individuals cannot be ruled out.Hepatic Impairment: Hepatic impairment influences the exposure of Eltrombopag. Reduce the initial dose of Eltrombopag in patients with chronic ITP (adult and pediatric patients 6 years and older only) or severe aplastic anemia who also have hepatic impairment (Child-Pugh Class A, B, C). No dosage adjustment is necessary for patients with chronic hepatitis C and hepatic impairment.Renal Impairment: No adjustment in the initial dose of Eltrombopag is needed for patients with renal impairment. Closely monitor patients with impaired renal function when administering Eltrombopag.Sku: 1736096953-1362
Elopag25 mg
₦192,500.00Original price was: ₦192,500.00.₦182,875.00Current price is: ₦182,875.00.₦192,500.00Original price was: ₦192,500.00.₦182,875.00Current price is: ₦182,875.00. Add to basket Quick View -
-
-
-
-
SaleElorim 13.9%Elorim 13.9% cream, 13.9% is indicated for the reduction of unwanted facial hair in women. Elorim 13.9% has only been studied on the face and adjacent involved areas under the chin of affected individuals. Usage should be limited to these areas of involvement.Theropeutic ClassHair Growth InhibitorPharmacologyEflornithine prevents hair growth by inhibiting the anagen phase of hair production. This occurs by eflornithine irreversibly binding (also called suicide inhibition) to ornithine decarboxylase (ODC) and physically preventing the natural substrate ornithine from accessing the active site.Dosage & Administration of Elorim 13.9%Adults: Apply a thin layer of Eflornithine cream, 13.9% to affected areas of the face and adjacent involved areas under the chin and rub in thoroughly. Do not wash treated area for at least 4 hours. Use twice daily at least 8 hours apart or as directed by a physician. The patient should continue to use hair removal techniques as needed in conjunction with Eflornithine (Wonica should be applied at least 5 minutes after hair removal). Cosmetics or sunscreens may be applied over treated areas after cream has dried.?Elderly: No apparent differences in safety were observed between older patients and younger patients.?Children: The safety and effectiveness of this product have not been established in pediatric patients less than 12 years of age.Dosage of Elorim 13.9%Adults: Apply a thin layer of Eflornithine cream, 13.9% to affected areas of the face and adjacent involved areas under the chin and rub in thoroughly. Do not wash treated area for at least 4 hours. Use twice daily at least 8 hours apart or as directed by a physician. The patient should continue to use hair removal techniques as needed in conjunction with Eflornithine (Wonica should be applied at least 5 minutes after hair removal). Cosmetics or sunscreens may be applied over treated areas after cream has dried.?Elderly: No apparent differences in safety were observed between older patients and younger patients.?Children: The safety and effectiveness of this product have not been established in pediatric patients less than 12 years of age.Interaction of Elorim 13.9%It is not known whether Eflornithine has any interaction with other topically applied drug products.ContraindicationsEflornithine is contraindicated in patients with a history of sensitivity to any components of the preparation.Side Effects of Elorim 13.9%Adverse events were primarily mild in intensity and generally resolved without medical treatment or discontinuation of Eflornithine. Side effects can include acne, barbae, pseudofolliculitis, stinging skin, headache, burning skin, dry skin, erythema (redness), pruritus (itching), tingling skin, dyspepsia, skin irritation, rash, alopecia, dizziness, folliculitis, hair ingrown, facial edema, anorexia, nausea, asthenia, vertigoPregnancy & LactationPregnancy Category C.?It is not known whether or not Elorim 13.9% is excreted in human milk. Caution should be exercised when Eflornithine is administered to a nursing woman.Precautions & WarningsFor external use only. Transient stinging or burning may occur when applied to abraded or broken skin.Overdose Effects of Elorim 13.9%Overdosage information with Eflornithine?is unavailable. However, if very high topical doses (e.g., multiple tubes per day) or oral ingestion has been encountered (a 30 gm tube contains 4.2 gm of Elorim 13.9%), the patient should be monitored, and appropriate supportive measures should be administered as necessary.Storage ConditionsStore at 25?C, excursions permitted to 15?C-30?C. Do not freeze.Drug ClassesHair Growth InhibitorMode Of ActionEflornithine prevents hair growth by inhibiting the anagen phase of hair production. This occurs by eflornithine irreversibly binding (also called suicide inhibition) to ornithine decarboxylase (ODC) and physically preventing the natural substrate ornithine from accessing the active site.PregnancyPregnancy Category C.?It is not known whether or not Elorim 13.9% is excreted in human milk. Caution should be exercised when Eflornithine is administered to a nursing woman.Sku: 1736097084-1399
Elorim13.9%
₦82,500.00Original price was: ₦82,500.00.₦74,250.00Current price is: ₦74,250.00.₦82,500.00Original price was: ₦82,500.00.₦74,250.00Current price is: ₦74,250.00. Add to basket Quick View -
SaleELORITE ? F CREAM (10g)Sku: 1728570280-1585
ELORITE ? F CREAM (10g)
₦4,200.00Original price was: ₦4,200.00.₦3,900.00Current price is: ₦3,900.00.₦4,200.00Original price was: ₦4,200.00.₦3,900.00Current price is: ₦3,900.00. Add to basket Quick View -
SaleELORITE CREAM (10g)Sku: 1728570277-1584
ELORITE CREAM (10g)
₦2,250.00Original price was: ₦2,250.00.₦2,100.00Current price is: ₦2,100.00.₦2,250.00Original price was: ₦2,250.00.₦2,100.00Current price is: ₦2,100.00. Add to basket Quick View -
SaleELOSONE ? HT CREAM (25g)Sku: 1728570275-1583
ELOSONE ? HT CREAM (25g)
₦4,350.00Original price was: ₦4,350.00.₦2,400.00Current price is: ₦2,400.00.₦4,350.00Original price was: ₦4,350.00.₦2,400.00Current price is: ₦2,400.00. Add to basket Quick View -
SaleSku: 1737123720-7051
ELOSONE CREAM
₦1,750.00Original price was: ₦1,750.00.₦1,443.75Current price is: ₦1,443.75.₦1,750.00Original price was: ₦1,750.00.₦1,443.75Current price is: ₦1,443.75. Add to basket Quick View -
SaleSku: 1737123693-7050
ELOSONE HT 15GM CREAM
₦3,625.00Original price was: ₦3,625.00.₦2,987.00Current price is: ₦2,987.00.₦3,625.00Original price was: ₦3,625.00.₦2,987.00Current price is: ₦2,987.00. Add to basket Quick View