-
PROfertil? Male Fertility Supplement Capsule, Pack of 60's 10 % ₦215,208.00 QTY: 1
-
ANAFLAM TH 4MG TAB 10`S ₦3,729.50 QTY: 1
-
Pankizol Tablet ₦520.00 QTY: 2
-
Macspacer Kit 1?S ₦68,000.00 QTY: 2
-
RAXITID 150MG TABLET ₦3,042.25 QTY: 1
-
Voglibite GM 1/0.2 Tablet ? 10Tab ₦8,250.00 QTY: 1
-
Benylin Chesty Cough Non-Drowsy Syrup, 150mls ₦6,850.00 QTY: 1
-
TEWHITE SOAP (75g) ₦2,600.00 QTY: 2
-
Mebo 0.25% Ointment 15 g ₦21,960.00 QTY: 3
-
Kelkin Gluten Free Porridge- Gluten Free Food for Coeliacs ₦22,700.00 QTY: 1
-
CIPLOX 250MG TAB 10`S ₦530.75 QTY: 1
-
GABAZEN CAP ₦6,187.50 QTY: 1
-
Aziderm 10% gel (15gm) ₦1,900.00 QTY: 1
-
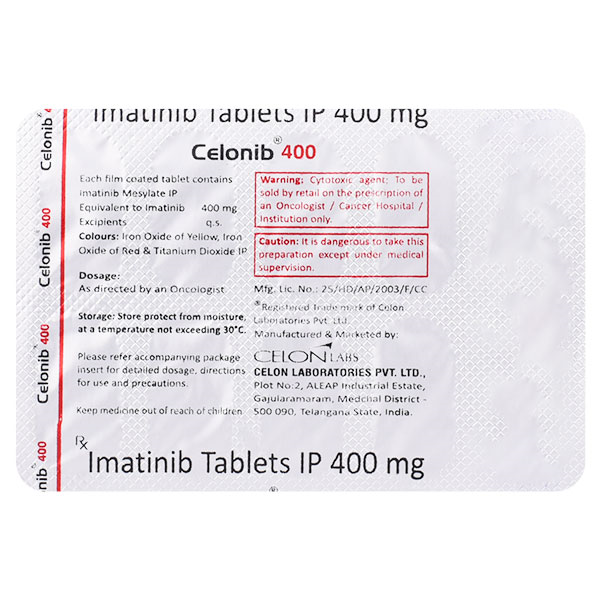
Celonib 400Mg Tablet 10?s ₦134,300.00 QTY: 1
-
DERINIDE 200MCG CFC FREE BOX OF 200MD METERED DOSE INHALER ₦9,054.75 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
33–48 of 4627 Results
-
SaleActrapid Penfill 100IU/mlTreatment of all type 1 diabetic patient. treatment for those with type 2 diabetes whose blood sugar cannot be sufficiently controlled by diet or oral hypoglycemic medications. for the initial stabilization of diabetes in people with diabetic ketoacidosis, hyperosmolar non-ketotic syndrome, and during stressful times like serious illnesses and major surgery for people with diabetes. gestational diabetes treatment.Theropeutic ClassRapid Acting InsulinPharmacologyThe ability of insulin to connect to receptors on muscle and fat cells and to simultaneously restrict the liver's ability to release glucose are what cause it to drop blood sugar levels. Long-acting insulin is called Insulatard. The action begins within one and a half hours, has its peak effect within four to twelve hours, and lasts for roughly twenty-four hours. The half-life of insulin in the blood is only a few minutes. As a result, only the features of an insulin preparation's absorption dictate its time-action profile. Many elements have an impact on this process (e.g. insulin dosage, injection route, and site, the thickness of subcutaneous fat, and type of diabetes).Dosage of Actrapid Penfill 100IU/mlUse your insulin exactly as directed by your doctor, adjusting the dosage as necessary. If you're unsure, ask your doctor, pharmacist, or nurse. Within 30 minutes of the injection, consume a meal or snack that contains carbs to prevent low blood sugar. If your doctor doesn't instruct you to, don't adjust your insulin. Your doctor may need to change your dose if you were switched from one brand or kind of insulin to another.Administration of Actrapid Penfill 100IU/mlfor usage beneath the skin. Typically, Insulatard is injected subcutaneously into the thigh. If more practical, the deltoid area, gluteal area, or abdominal wall may also be employed. In comparison to the other injection locations, the thigh's subcutaneous injection causes a slower and less varied absorption. Unintentional intramuscular injection is less likely when the skin fold is injected into. To ensure that the entire dosage is injected, keep the needle subcutaneously for at least 6 seconds. To prevent lipodystrophy, injection sites should be rotated within anatomical regions. Never inject insulin suspensions intravenously. A package booklet for Insulatard contains comprehensive usage instructions that must be followed. The vials are meant to be used with equivalent unit insulin syringes. When two types of insulin are mixed, draw the amount of fast-acting insulin first, followed by the amount of long-acting insulin.Side Effects of Actrapid Penfill 100IU/mlHypoglycemia, or low blood sugar, is a relatively frequent adverse consequence. More than one in ten persons might be impacted. Low blood sugar symptoms include cold perspiration, chilly pale skin, headache, rapid heartbeat, nausea, extreme hunger, brief changes in vision, fatigue, unusual weakness, anxiety or tremor, feeling agitated, feeling confused, and difficulty focusing.Pregnancy & LactationBefore using this medication, consult your doctor if you are pregnant, suspect you may be pregnant, or are planning a pregnancy. You can use this insulin while you're pregnant. You might need to adjust your insulin dosage both during pregnancy and after birth. Careful control of your diabetes, particularly prevention of hypoglycaemia, is important for the health of your baby. The use of this insulin during breastfeeding is not restricted.Precautions & WarningsIf you experience issues with your adrenal, pituitary, thyroid, or liver glands. If you decide to vary your typical diet or engage in more exercise than usual, as these actions may have an impact on your blood sugar level. Continue taking your insulin and see a doctor if you feel sick. Traveling across time zones when visiting another country could change your demand for insulin and the timing of this.Storage ConditionsKeep refrigerated at 2?C to 8?C. Avoid touching the cooling device. Avoid freezing.Use In Special PopulationsUsage in children and adolescents: Children and adolescents may use this. Usage with specific patient populations You should check your blood sugar more frequently and talk to your doctor about adjusting your insulin dosage if you have impaired kidney or liver function or if you are over 65.Sku: 1736103616-3317
Actrapid Penfill100IU/ml
₦25,300.00Original price was: ₦25,300.00.₦24,035.00Current price is: ₦24,035.00.₦25,300.00Original price was: ₦25,300.00.₦24,035.00Current price is: ₦24,035.00. Add to basket Quick View -
SaleActrapid Vial 100IU/10 mlTreatment of all type 1 diabetic patient. treatment for those with type 2 diabetes whose blood sugar cannot be sufficiently controlled by diet or oral hypoglycemic medications. for the initial stabilization of diabetes in people with diabetic ketoacidosis, hyperosmolar non-ketotic syndrome, and during stressful times like serious illnesses and major surgery for people with diabetes. gestational diabetes treatment.Theropeutic ClassRapid Acting InsulinPharmacologyThe ability of insulin to connect to receptors on muscle and fat cells and to simultaneously restrict the liver's ability to release glucose are what cause it to drop blood sugar levels. Long-acting insulin is called Insulatard. The action begins within one and a half hours, has its peak effect within four to twelve hours, and lasts for roughly twenty-four hours. The half-life of insulin in the blood is only a few minutes. As a result, only the features of an insulin preparation's absorption dictate its time-action profile. Many elements have an impact on this process (e.g. insulin dosage, injection route, and site, the thickness of subcutaneous fat, and type of diabetes).Dosage of Actrapid Vial 100IU/10 mlUse your insulin exactly as directed by your doctor, adjusting the dosage as necessary. If you're unsure, ask your doctor, pharmacist, or nurse. Within 30 minutes of the injection, consume a meal or snack that contains carbs to prevent low blood sugar. If your doctor doesn't instruct you to, don't adjust your insulin. Your doctor may need to change your dose if you were switched from one brand or kind of insulin to another.Administration of Actrapid Vial 100IU/10 mlfor usage beneath the skin. Typically, Insulatard is injected subcutaneously into the thigh. If more practical, the deltoid area, gluteal area, or abdominal wall may also be employed. In comparison to the other injection locations, the thigh's subcutaneous injection causes a slower and less varied absorption. Unintentional intramuscular injection is less likely when the skin fold is injected into. To ensure that the entire dosage is injected, keep the needle subcutaneously for at least 6 seconds. To prevent lipodystrophy, injection sites should be rotated within anatomical regions. Never inject insulin suspensions intravenously. A package booklet for Insulatard contains comprehensive usage instructions that must be followed. The vials are meant to be used with equivalent unit insulin syringes. When two types of insulin are mixed, draw the amount of fast-acting insulin first, followed by the amount of long-acting insulin.Side Effects of Actrapid Vial 100IU/10 mlHypoglycemia, or low blood sugar, is a relatively frequent adverse consequence. More than one in ten persons might be impacted. Low blood sugar symptoms include cold perspiration, chilly pale skin, headache, rapid heartbeat, nausea, extreme hunger, brief changes in vision, fatigue, unusual weakness, anxiety or tremor, feeling agitated, feeling confused, and difficulty focusing.Pregnancy & LactationBefore using this medication, consult your doctor if you are pregnant, suspect you may be pregnant, or are planning a pregnancy. You can use this insulin while you're pregnant. You might need to adjust your insulin dosage both during pregnancy and after birth. Careful control of your diabetes, particularly prevention of hypoglycaemia, is important for the health of your baby. The use of this insulin during breastfeeding is not restricted.Precautions & WarningsIf you experience issues with your adrenal, pituitary, thyroid, or liver glands. If you decide to vary your typical diet or engage in more exercise than usual, as these actions may have an impact on your blood sugar level. Continue taking your insulin and see a doctor if you feel sick. Traveling across time zones when visiting another country could change your demand for insulin and the timing of this.Storage ConditionsKeep refrigerated at 2?C to 8?C. Avoid touching the cooling device. Avoid freezing.Use In Special PopulationsUsage in children and adolescents: Children and adolescents may use this. Usage with specific patient populations You should check your blood sugar more frequently and talk to your doctor about adjusting your insulin dosage if you have impaired kidney or liver function or if you are over 65.Sku: 1736107732-4537
Actrapid Vial100IU/10 ml
₦22,825.00Original price was: ₦22,825.00.₦20,542.50Current price is: ₦20,542.50.₦22,825.00Original price was: ₦22,825.00.₦20,542.50Current price is: ₦20,542.50. Add to basket Quick View -
SaleAcuren 25 mgEdema associated with congestive heart failure, hepatic cirrhosis, premenstrual tension and oedema due to various forms of renal dysfunction (i.e. nephrotic syndrome, acute glomerulonephritis, chronic renal failure). Hypertension, either alone or as an adjunct to other antihypertensive drugs.Theropeutic ClassThiazide diuretics & related drugsPharmacologyThiazides such as Acuren 25 mg promote water loss from the body (diuretics). They inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.Dosage & Administration of Acuren 25 mgAdults- For Edema: The usual adult dosage is 25 to 100 mg daily as a single or divided dose.For Control of Hypertension: The usual initial dose in adults is 25 mg daily given as a single dose. The dose may be increased to 50 mg daily, given as a single or two divided doses. Doses above 50 mg are often associated with marked reductions in serum potassium. In some patients (especially the elderly) an initial dose of 12.5 mg daily may be sufficient. Infants and children- For diuresis and for control of hypertension: The usual pediatric dosage is 1 to 2 mg/kg/day in single or two divided doses, not to exceed 37.5 mg per day in infants up to 2 years of age or 100 mg per day in children 2 to 12 years of age. In infants less than 6 months of age, doses up to 3 mg/kg/day in two divided doses may be required.Dosage of Acuren 25 mgEdema: initially 25 to 50 mg daily, reduced for maintenance if possible; maximum 100 mg daily.Hypertension: 25 mg daily, increased to 50 mg daily if necessary.Elderly: in some patients, especially the elderly an initial dose of 12.5 mg daily may be sufficient.Children: An initial dose for children has been 1 to 2 mg per kg body-weight in 2 divided doses. Infants under 6 months may need doses up to 3 mg per kg daily.Interaction of Acuren 25 mgAlcohol, barbiturates or narcotics: Co-administration may potentiate orthostatic hypotension. Oral and parenteral antidiabetic drugs may require adjustment of dosage with concurrent use. Other antihypertensive drugs may have an additive effect. Discontinuation of diuretic therapy 2-3 days before the initiation of treatment with an ACE inhibitor may reduce the likelihood of first-dose hypotension. The antihypertensive effect of the drug may be enhanced in the post-sympathectomy patient.Cholestyramine and colestipol resin: Absorption of Acuren 25 mg is impaired in the presence of anionic exchange resin. Single doses of either cholestyramine or colestipol resins bind the Acuren 25 mg and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively. Corticosteroids or ACTH may intensify any Thiazide-induced electrolyte depletion, particularly hypokalaemia. Pressor amines such as adrenaline may show decreased arterial responsiveness when used with Acuren 25 mg, but this reaction is not enough to preclude their therapeutic usefulness. Non-depolarising muscle relaxants such as tubocurarine may possibly interact with Acuren 25 mg to increase muscle relaxation. Non-steroidal anti-inflammatory drugs may attenuate the diuretic and antihypertensive effects of diuretics.Drug/laboratory tests: Because thiazides may affect calcium metabolism, Acuren 25 mg may interfere with tests for parathyroid function.ContraindicationsAnuria, hypersensitivity to Acuren 25 mg or to other sulphonamide-derived drugs, severe renal or hepatic failure, Addison?s disease, hypercalcemia, concurrent lithium therapy.Side Effects of Acuren 25 mgGastro-intestinal system: Anorexia, gastric irritation, nausea, vomiting, cramps, diarrhoea, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis, salivary gland inflammation. Central nervous system: Dizziness, vertigo, paraesthesiae, headache, yellow vision.Heamatological: Leucopenia, agranulocytosis, thrombocytopenia, aplastic anaemia,?haemolytic anaemia.Cardiovascular: Hypotension, including orthostatic hypotension.Hypersensitivity: Purpura, photosensitivity, rash, urticaria, necrotising angiitis (vasculitis, cutaneous vasculitis), fever, respiratiory distress including pneumonitis and pulmonary oedema, anaphylactic reactions, toxic epidermal necrolysis.Metabolic: Hyperglycaemia, glycosuria, hyperuricaema, electrolyte imbalance including hyponatraemia and hypokalaemia.Renal: Renal dysfunction, interstitial nephritis, renal failure.Other: Muscle spasm, weakness, restlessness, transient blurred vision, impotence. Whenever side-effects are moderate to severe, thiazide dosage should be reduced or therapy was withdrawn.Pregnancy & LactationPregnancy: Evidence of fetal risk in Acuren 25 mg therapy is found, but it is indicated if benefits outweigh risks. Thiazides are indicated in pregnancy when edema is due to pathologic causes.Lactation: Neonatal side effects have been seen incase of Acuren 25 mg therapy and therefore it is not recommended.Precautions & WarningsPatients should be carefully monitored for signs of fluid and electrolyte imbalance (hyponatraemia, hypochloraemic alkalosis, hypokalaemia and hypomagnesaemia). It is particularly important to make serum and urine electrolyte determinations when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance include: dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, seizures, confusion, muscle pains or cramps, muscle fatigue, hypotension, oliguria, tachycardia, and gastro-intestinal disturbances such as nausea and vomiting. Hypokalaemia may develop, especially with brisk diuresis, when severe cirrhosis is present, or after prolonged therapy. Hypokalaemia can sensitise or exaggerate the response of the heart to the toxic effects of digitalis (e.g. increased ventricular irritability). Sensitivity reactions may occur in patients with or without history of allergy or bronchial asthma. Hypokalaemia may be avoided or treated in the adult by concurrent use of amiloride hydrochloride, a potassium conserving agent. It may also be avoided by giving potassium chloride or foods with a high potassium content. Diuretic-induced hyponatraemia is usually mild and asymptomatic. Dilutional hyponatraemia may occur in oedematous patients in hot weather; and, except in rare instances when hyponatraemia is life-threatening, appropriate therapy is water restriction rather than administration of salt. Thiazides may decrease serum protein bound iodine levels without signs of thyroid disturbances. Thiazides may decrease urinary calcium excretion, and may also cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Thiazides should be discontinued before carrying out tests for parathyroid function. When creatinine clearance falls below 30ml/min, thiazide diuretics become ineffective. Uraemia may be precipitated or increased by chlorothiazide. Cumulative effects of the drug may develop in patients with impaired renal function. If increasing uraemia and oliguria occur during treatment of renal disease, Acuren 25 mg should be discontinued. Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma. Hyperuricaemia may occur, or gout may be precipitated, in certain patients receiving thiazide therapy. Thaizide therapy may impair glucose tolerance. Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy. The possibility of exacerbation or activation of systemic lupus erythematosus has been reported. Latent diabetes may become manifest during thiazide administration.Overdose Effects of Acuren 25 mgThe most common signs and symptoms observed are those caused by electrolyte depletion (hypokalaemia, hypochloraemia, hyponatraemia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalaemia may accentuate cardiac arrhythmias. In the event of overdosage, symptomatic and supportive measures should be employed. If ingestion is recent, emesis should be induced or gastric lavage performed. Dehydration, electrolyte imbalance, hepatic coma and hypotension should be corrected by established methods. If required, give oxygen or artificial respiration for respiratory impairment.Storage ConditionsKeep below 30?C temperature, away from light & moisture. Keep out of the reach of children.Use In Special PopulationsElderly: in some patients specially the elderly an initial dose of 12.5 mg daily may be?sufficient.Children: An initial dose for children has been 1 to 2 mg per kg body-weight in 2 divided?doses. Infants under 6 months may need doses upto 3 mg per kg daily.Drug ClassesThiazide diuretics & related drugsMode Of ActionThiazides such as Acuren 25 mg promote water loss from the body (diuretics). They inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.PregnancyUse in pregnancy: Thiazides cross the placental barrier and appear in cord blood. The use of Acuren 25 mg when pregnancy is present or suspected requires, therefore, that the benefits of the drug be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions, which have occurred in the adult. The routine use of diuretics in otherwise healthy pregnant women with or without mild oedema is not recommended, because their use may be associated with hypovolaemia, increased blood viscosity and decreased placental perfusion.Use in breastfeeding mothers: Thiazides appear in breast milk. If use of the drug is deemed essential, the patient should stop breast-feeding.Sku: 1736101834-2805
Acuren25 mg
₦38.50Original price was: ₦38.50.₦34.65Current price is: ₦34.65. -
SaleAdaben Duo 0.1%+2.5%Adapalene & Benzoyl peroxide gel is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.CompositionEach gram of Gel contains Adapalene BP 1 mg and Benzoyl peroxide BP 25 mg.Theropeutic ClassTopical retinoid and related preparationsPharmacologyAdapalene & Benzoyl peroxide gel is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older. Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that Adapalene is a modulator of cellular differentiation, keratinization and inflammatory processes. Adapalene binds with specific retinoic acid nuclear receptors that normalize the differentiation of follicular epithelial cells resulting in decreased microcomedone formation. Benzoyl peroxide is an oxidizing agent with bacteriocidal and keratolytic effects.Dosage & Administration of Adaben Duo 0.1%+2.5%Apply a thin film of Adapalene & Benzoyl peroxide gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes. Adapalene & Benzoyl peroxide is not for oral, ophthalmic, or intravaginal use.Dosage of Adaben Duo 0.1%+2.5%Apply a thin film of Adapalene & Benzoyl peroxide gel to affected areas of the face and/or trunk once daily after washing. Use a pea-sized amount for each area of the face (e.g., forehead, chin, each cheek). Avoid the eyes, lips and mucous membranes. Adapalene & Benzoyl peroxide is not for oral, ophthalmic, or intravaginal use.Pediatric use: The safety and effectiveness of Adapalene and Benzoyl peroxide gel in pediatric patients under the age of 12 years have not been established.Interaction of Adaben Duo 0.1%+2.5%Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. No formal drug-drug interaction studies were conducted.ContraindicationsShould not be administered to individuals who are hypersensitive to any of its component.Side Effects of Adaben Duo 0.1%+2.5%Erythema, scaling, dryness, and stinging/ burning may occur. Most commonly reported adverse events are dry skin, contact dermatitis, application site burning, application site irritation, and skin irritation.Pregnancy & LactationThere are no well-controlled trials in pregnant women treated with Adapalene and Benzoyl peroxide. Animal reproduction studies have not been conducted with the combination gel or Benzoyl peroxide. Furthermore, such studies are not always predictive of human response; therefore, this preparation should be used during pregnancy only if the potential benefit justifies the risk to the fetus. It is not known whether Adapalene or Benzoyl peroxide is excreted in human milk following use. Caution should be exercised when administered to a nursing woman.Precautions & WarningsAvoid exposure to sunlight and sunlamps. Wear sunscreen when sun exposure cannot be avoided.Storage ConditionsStore in a cool (below 25?C) and dry place protected from light and moisture. Keep out of the reach of children. Keep the tube tightly closed after use.Use In Special PopulationsSafety and effectiveness of Adapalene and Benzoyl peroxide gel in pediatric patients under the age of 12 years have not been established.Drug ClassesTopical retinoid and related preparationsMode Of ActionAdapalene & Benzoyl peroxide gel is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older. Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that Adapalene is a modulator of cellular differentiation, keratinization and inflammatory processes. Adapalene binds with specific retinoic acid nuclear receptors that normalize the differentiation of follicular epithelial cells resulting in decreased microcomedone formation. Benzoyl peroxide is an oxidizing agent with bacteriocidal and keratolytic effects.PregnancyThere are no well-controlled trials in pregnant women treated with Adapalene and Benzoyl peroxide. Animal reproduction studies have not been conducted with the combination gel or Benzoyl peroxide. Furthermore, such studies are not always predictive of human response; therefore, this preparation should be used during pregnancy only if the potential benefit justifies the risk to the fetus. It is not known whether Adapalene or Benzoyl peroxide is excreted in human milk following use. Caution should be exercised when administered to a nursing woman.Sku: 1736101469-2701
Adaben Duo0.1%+2.5%
₦11,000.00Original price was: ₦11,000.00.₦9,900.00Current price is: ₦9,900.00.₦11,000.00Original price was: ₦11,000.00.₦9,900.00Current price is: ₦9,900.00. Add to basket Quick View -
SaleAdafil 10 mgAdafil 10 mg is indicated in- Erectile Dysfunction (ED) Benign Prostatic Hyperplasia (BPH) Both Erectile Dysfunction and signs and symptoms of Benign Prostatic Hyperplasia Theropeutic ClassDrugs for Erectile DysfunctionPharmacologyAdafil 10 mg is a selective phosphodiesterase type 5 (PDE5) inhibitor. Inhibition of PDE5 increases cGMP in smooth muscle cells. cGMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum, causing penile erection. PDE5 also is present in smooth muscles of the prostate and bladder wall. Inhibiting PDE5 increases cGMP concentrations leading to relaxation of smooth muscle in the prostate and bladder. Smooth muscle relaxation may improve blood flow to the urinary tract and widen the opening of the bladder neck, resulting in improved voiding.Dosage & Administration of Adafil 10 mgErectile Dysfunction: For most patients the recommended starting dose is 10 mg. The dose may be increased to 20 mg or decreased to 5 mg based on requirement. The maximum dosing frequency is once daily. Adafil 10 mg is effective for up to 36 hours.Benign prostatic hyperplasia: The recommended dose is 5 mg taken at the same time every day.Combined Erectile Dysfunction and Benign prostatic hyperplasia: The recommended dose is 5 mg at the same time every day.Dosage of Adafil 10 mgErectile Dysfunction: For most patients the recommended starting dose is 10 mg. The dose may be increased to 20 mg or decreased to 5 mg based on requirement. The maximum dosing frequency is once daily. Adafil 10 mg is effective for up to 36 hours.Benign prostatic hyperplasia: The recommended dose is 5 mg taken at the same time every day.Combined Erectile Dysfunction and Benign prostatic hyperplasia: The recommended dose is 5 mg at the same time every day.Interaction of Adafil 10 mgMay interact with Nitrates for example, Isosorbide, Nitroglycerin, Alpha adrenergic blockers, Antihypertensives, Alcohol, Antacids (magnesuim hydroxide/aluminum hydroxide), Ketoconazole, Ritonavir, Erythromycin, Itraconazole, Grapefruit juice, other HIV protease inhibitors, Rifampin, Carbamazepine, Phenytoin & Phenobarbital.Contraindications Use of Nitrates (for example, Nitroglycerine, Isosorbide): may increase hypotensive effects of Nitrates Hypersensitivity reactions to Adafil 10 mg Side Effects of Adafil 10 mgHeadache, Dyspepsia, Back pain, Myalgia, Nasal pharyngitis, Nasal congestion are common side effects. Change in Color Vision, Sudden vision loss, Hearing loss, Stevens-Johnson Syndrome, Exfoliative dermatitis, Angina, Stroke, Myocardial infarction, Severe hypotension, Tachycardia may also occur rarely.Pregnancy & LactationAdafil 10 mg has been assigned to pregnancy category B by the USFDA. Adafil 10 mg is only recommended for use during pregnancy when benefit outweighs risk. There are no data on the excretion of Adafil 10 mg in human milk. Caution should be used when administering Adafil 10 mg to nursing women.Precautions & WarningsAngina, renal impairment, hepatic impairment, bleeding concomitant with Nitrates, Alpha Blockers, Alcohol, CYP3A4 Inhibitors (for example, Ritonavir, Ketoconazole, Itraconazole), other PDE5 inhibitors precaution should be taken in all these conditions.Storage ConditionsKeep in a dry place, away from light and heat. Keep out of the reach of children.Drug ClassesDrugs for Erectile DysfunctionMode Of ActionAdafil 10 mg is a selective phosphodiesterase type 5 (PDE5) inhibitor. Inhibition of PDE5 increases cGMP in smooth muscle cells. cGMP causes smooth muscle relaxation and increased blood flow into the corpus cavernosum, causing penile erection. PDE5 also is present in smooth muscles of the prostate and bladder wall. Inhibiting PDE5 increases cGMP concentrations leading to relaxation of smooth muscle in the prostate and bladder. Smooth muscle relaxation may improve blood flow to the urinary tract and widen the opening of the bladder neck, resulting in improved voiding.PregnancyAdafil 10 mg has been assigned to pregnancy category B by the USFDA. Adafil 10 mg is only recommended for use during pregnancy when benefit outweighs risk. There are no data on the excretion of Adafil 10 mg in human milk. Caution should be used when administering Adafil 10 mg to nursing women.Sku: 1736105802-3959
Adafil10 mg
₦1,925.00Original price was: ₦1,925.00.₦1,732.50Current price is: ₦1,732.50.₦1,925.00Original price was: ₦1,925.00.₦1,732.50Current price is: ₦1,732.50. Add to basket Quick View -
SaleAdam 33 5 mgRheumatic Disorders: Psoriatic arthritis, rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosing spondylitis, acute and subacute bursitis, acute nonspecific tenosynovitis, acute gouty arthritis, post-traumatic osteoarthritis. Endocrine Disorders: Primary or secondary adrenocortical insufficiency, congenital adrenal hyperplasia, nonsuppurative thyroiditis, hypercalcemia associated with cancer. Dermatologic Diseases: Pemphigus, bullous dermatitis herpetiformis, severe erythema multiforme, exfoliative dermatitis, mycosis fungoides, severe psoriasis. Allergic States: Seasonal or perennial allergic rhinitis, bronchial asthma, contact dermatitis, atopic dermatitis, serum sickness, drug hypersensitivity reactions.Respiratory Diseases: Symptomatic sarcoidosis, berylliosis, fulminating, aspiration pneumonitis. Hematologic Disorders: Idiopathic thrombocytopenic purpura, secondary thrombocytopenia, acquired (autoimmune) hemolytic anemia, erythroblastopenia (RBC anemia). Edematous States: To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus. Gastrointestinal Diseases: Ulcerative colitis, regional enteritis.Theropeutic ClassGlucocorticoidsPharmacologyAdam 33 5 mg decreases inflammation by inhibition of migration of polymorphonuclear leukocytes and reversal of increased capillary permeability. It suppresses the immune system by reducing the activity and production of the lymphocytes and eosinophils.Dosage & Administration of Adam 33 5 mgThe initial dose may vary from 5 mg to 60 mg per day depending on the specific disease. After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. Constant monitoring is needed in regard to drug dosage. If after long-term therapy the drug is to be stopped, it is recommended that it should be withdrawn gradually rather than abruptly. Multiple Sclerosis: In the treatment of acute exacerbations of multiple sclerosis daily doses of 200 mg of Adam 33 5 mg for a week followed by 80 mg every other day for 1 month have been shown to be effective.Dosage of Adam 33 5 mgAdult-Nephrotic Syndrome: Initial: 2 mg/kg/day (maximum 80 mg/day) in divided doses 3 to 4 times/day until urine is protein free for 3 consecutive days (maximum: 28 days); followed by 1 to 1.5 mg/kg/dose given every other day for 4 weeks. Maintenance dose: 0.5 to 1 mg/kg/ dose given every other day for 3 to 6 months. Anti-inflammatory: 5 to 60 mg per day in divided doses 1 to 4 times/day.Acute Asthma: 40-60 mg/day PO in single daily dose or divided q12 hr for 3-10 days.Allergic Conditions: Day 1: 10 mg PO before breakfast, 5 mg after lunch and after dinner, and 10 mg at bedtime. Day 2: 5 mg PO before breakfast, after lunch, and after dinner and 10 mg at bedtime. Day 3: 5 mg PO before breakfast, after lunch, after dinner, and at bedtime. Day 4: 5 mg PO before breakfast, after lunch, and at bedtime. Day 5: 5 mg PO before breakfast and at bedtime. Day 6: 5 mg PO before breakfast. Pediatric-Asthma: 1 year: Acute: 10 mg orally every 12 hours. Maintenance: 10 mg orally every other day. 1 to 4 years: Acute: 20 mg orally every 12 hours. Maintenance: 20 mg orally every other day. 5 to 12 years: Acute: 30 mg orally every 12 hours. Maintenance: 30 mg orally every other day. 12 years: Acute: 40 mg orally every 12 hours. Maintenance: 40 mg orally every other day. Anti-inflammatory: 0.05 to 2 mg/kg/day divided 1 to 4 times/day.Immunosuppression: 0.05 to 2 mg/kg/day divided 1 to 4 times/day.Interaction of Adam 33 5 mgThe efficacy of Adam 33 5 mg is reduced by Aminoglutethimide, Antacids, Barbiturates, Carbamazepine, Griseofulvin, Mitotane, Phenylbutazone, Phenytoin, Primidone and Rifampin. Adam 33 5 mg reduces the amount of potassium in the blood. Digitalis can cause Cardiac arrhythmias if hypokalemia occurs. Immunization should be done very carefully.ContraindicationsSystemic infections unless specific anti-infective therapy is employed. Hypersensitivity to any ingredient. Ocular herpes simplex because of possible perforation.Side Effects of Adam 33 5 mgCommon side effects include increased appetite, indigestion, nervousness or restlessness. Less frequent or rare side effects are darkening or lightening of skin color, dizziness or lightheadedness, flushing of face or cheeks, hiccups, increased sweating, the sensation of spinning.Pregnancy & LactationSince adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers or women of childbearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy, should be carefully observed for signs of hypoadrenalism. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretionPrecautions & WarningsPrecaution has to be taken in diabetes, hypertension, Psychological disturbances, osteoporosis, post-menopausal women, pregnancy and in chronic nephritis. Long-term use of Adam 33 5 mg can cause Cushing's habitus, hyperglycemia, muscular weakness, increased susceptibility to infection, delayed wound healing, and psychiatric disturbances.Overdose Effects of Adam 33 5 mgAdverse effects related to prednisone normally develop only after prolonged use of doses in excess of the normal physiological requirement. Treatment is symptomatic and where possible the prednisone dose should be reduced gradually.Storage ConditionsStore in a cool and dry place, protected from light. Keep out of the reach of the children.Drug ClassesGlucocorticoidsMode Of ActionAdam 33 5 mg is a synthetic adrenocortical drug with predominantly glucocorticoid properties. It directly inhibits the action of the Phospholipase A2 enzyme which is responsible for the production of different inflammatory mediators like Leukotrienes, SRS-A, Prostaglandins etc. Adam 33 5 mg is rapidly and well absorbed from the Gl tract following oral administration. the medicine is 70- 90% protein-bound in the plasma and it is eliminated from the plasma with a half-life of 2 to 4 hours. It is metabolized mainly in the liver and excreted in the urine.Sku: 1736097619-1554
Adam 335 mg
₦95.15Original price was: ₦95.15.₦85.80Current price is: ₦85.80. -
SaleAdarbi 40 mgAdarbi 40 mg is indicated for the treatment of hypertension to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily stroke and myocardial infarction. Adarbi 40 mg may be used either alone or in combination with other antihypertensive agents.Theropeutic ClassAngiotensin-ll receptor blockerPharmacologyAdarbi 40 mg, a prodrug, is hydrolyzed to Azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist. Azilsartan blocks the vasoconstrictor and aldosterone secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland.Dosage & Administration of Adarbi 40 mgThe recommended dose in adults is 80 mg taken orally once daily. Consider a Starting dose of 40 mg for patients who are treated with high doses of diuretics. If blood pressure is not controlled with Azilsartan alone, additional blood pressure reduction can be achieved by taking Azilsartan with other antihypertensive agents.Dosage of Adarbi 40 mgThe recommended dose in adults is 80 mg taken orally once daily. Consider a Starting dose of 40 mg for patients who are treated with high doses of diuretics. If blood pressure is not controlled with Azilsartan alone, additional blood pressure reduction can be achieved by taking Azilsartan with other antihypertensive agents.Interaction of Adarbi 40 mgNo drug interactions have been observed in studies of Adarbi 40 mg or Azilsartan given with amlodipine, antacids, chlorthalidone, digoxin, fluconazole, glyburide, ketoconazole, metformin, pioglitazone and warfarin. The antihypertensive effect of Azilsartan may be attenuated by the non-steroidal anti-inflammatory drugs including selective COX-2 inhibitors. Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors or aliskiren is associated with increased risks of hypotension, hyperkalemia and changes in renal function.ContraindicationsIt is contraindicated to co-administer Aliskiren with Azilsartan in patients with Diabetes.Side Effects of Adarbi 40 mgThe most common adverse reaction in adults is diarrhea. The other side effects are nausea, asthenia, fatigue, muscle spasm, dizziness and cough.Pregnancy & LactationPregnancy Category D. The risk to the fetus increases if Adarbi 40 mg is administered during the second or third trimesters of pregnancy. It is not known whether Adarbi 40 mg is excreted in human milk, as many drugs are excreted in human milk and because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.Precautions & WarningsUse of Adarbi 40 mg during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. In patients who are intravascularly volume-depleted (e.g., those treated with high-dose diuretics), symptomatic hypotension may occur. Changes in renal function including renal failure has been reported in renal impaired patient.Overdose Effects of Adarbi 40 mgLimited data are available related to overdose in humans. In the event of and overdose, supportive therapy should be instituted as dictated by the patient?s clinical status. Azilsartan is not dialyzable.Storage Conditionskeep in a dry place away from light and heat. Keep out of the reach of children.Use In Special PopulationsSafety and effectiveness in pediatric patients under 18 years of ages have not been established.Drug ClassesAngiotensin-ll receptor blockerMode Of ActionAngiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzymes (ACE, kinase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with efects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Azilsartan blocks the vasoconstrictor and aldosteronesecreting efects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathway for angiotensin II synthesis. An AT2 receptor is also found in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Azilsartan has more than a 10,000-fold greater afnity for the AT1 receptor than for the AT2 receptor.Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction catalyzed by ACE. Because azilsartan does not inhibit ACE (kinase II), it should not afect bradykinin levels. Whether this diference has clinical relevance is not yet known. Azilsartan does not bind to or block other receptors or ion channels known to be important in cardiovascular regulation. Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the efect of azilsartan on blood pressure.Azilsartan inhibits the pressor efects of an angiotensin II infusion in a dose-related manner. An azilsartan single dose equivalent to 32 mg Adarbi 40 mg inhibited the maximal pressor efect by approximately 90% at peak, and approximately 60% at 24 hours. Plasma angiotensin I and II concentrations and plasma renin activity increased while plasma aldosterone concentrations decreased after single and repeated administration of Azilsartan to healthy subjects; no clinically signifcant efects on serum potassium or sodium were observed.Absorption: Azilsartan medoxomil is hydrolyzed to azilsartan, the active metabolite, in the gastrointestinal tract during absorption. Azilsartan medoxomil is not detected in plasma after oral administration. Dose proportionality in exposure was established for azilsartan in the Adarbi 40 mg dose range of 20 mg to 320 mg after single or multiple dosing. The estimated absolute bioavailability of azilsartan following administration of Adarbi 40 mg is approximately 60%. After oral administration of Adarbi 40 mg, peak plasma concentrations (Cmax) of azilsartan are reached within 1.5 to 3 hours. Food does not afect the bioavailability of azilsartan.Distribution: The volume of distribution of azilsartan is approximately 16L. Azilsartan is highly bound to human plasma proteins (>99%), mainly serum albumin. Protein binding is constant at azilsartan plasma concentrations well above the range achieved with recommended doses. In rats, minimal azilsartan-associated radioactivity crossed the blood-brain barrier. Azilsartan passed across the placental barrier in pregnant rats and was distributed to the fetus.Metabolism and Elimination: Azilsartan is metabolized to two primary metabolites. The major metabolite in plasma is formed by O-dealkylation, referred to as metabolite M-II, and the minor metabolite is formed by decarboxylation, referred to as metabolite M-I. Systemic exposures to the major and minor metabolites in humans were approximately 50% and less than 1% of azilsartan, respectively. M-I and M-II do not contribute to the pharmacologic activity of Azilsartan. The major enzyme responsible for azilsartan metabolism is CYP2C9.PregnancyPregnancy Category D. The risk to the fetus increases if Adarbi 40 mg is administered during the second or third trimesters of pregnancy. It is not known whether Adarbi 40 mg is excreted in human milk, as many drugs are excreted in human milk and because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.Pediatric UsesPediatric Use: Neonates with a history of in utero exposure to azilsartan. If oliguria or hypotension occurs, support blood pressure and renal function. Exchange transfusions or dialysis may be required. Safety and effectiveness in pediatric patients under 18 years of age have not been established.Geriatric Use: No dose adjustment with Azilsartan is necessary in elderly patients. Of the total patients in clinical studies with Azilsartan, 26% were elderly (65 years of age and older); 5% were 75 years of age and older. Abnormally high serum creatinine values were more likely to be reported for patients age 75 year or older. No other differences in safety or effectiveness were observed between elderly patients and younger patients, but the greater sensitivity of some older individuals cannot be ruled out.Renal Impairment: Dose adjustment is not required in patients with mild-to-severe renal impairment or end-stage renal disease. Patients with moderate to severe renal impairment are more likely to report abnormally high serum creatinine values.Hepatic Impairment: No dose adjustment is necessary for subjects with mild or moderate hepatic impairment. Azilsartan has not been studied in patients with severe hepatic impairment.Sku: 1736101507-2712
Adarbi40 mg
₦770.00Original price was: ₦770.00.₦693.00Current price is: ₦693.00. -
SaleAdegra 50 mgSildenafil is indicated for the treatment of erectile dysfunction and pulmonary arterial hypertension.Theropeutic ClassDrugs for Erectile DysfunctionPharmacologySildenafil is a selective inhibitor of cyclic Guanosine Monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5) used for treatment of erectile dysfunction. Danafil (Sildenafil) enhances the effect of nitric oxide (NO) by inhibiting phosphodiesterase type 5 (PDE5), which is responsible for degradation of cGMP in the corpus cavernosum that results in smooth muscle relaxation and inflow of blood to the corpus cavernosum.Dosage of Adegra 50 mgErectile dysfunction: For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, Sildenafil may be taken anywhere from 4 hours to 0.5 hour before sexual activity. Based on effectiveness and toleration, the dose may be increased to a maximum recommended dose of 100 mg or decreased to 25 mg. The maximum recommended dosing frequency is once per day.The following factors are associated with increased plasma levels of Sildenafil: age >65, hepatic impairment, severe renal impairment, and concomitant use of potent cytochrome P450 3A4 inhibitors (ketoconazole, itraconazole, erythromycin, saquinavir). Since higher plasma levels may increase both the efficacy and incidence of adverse events, a starting dose of 25 mg should be considered in these patients. Sildenafil was shown to potentiate the hypotensive effects of nitrates and its administration in patients who use nitric oxide donors or nitrates in any form is therefore contraindicated. When Sildenafil is co-administered with an alpha-blocker, patients should be stable on alphablocker therapy prior to initiating Sildenafil treatment and Sildenafil should be initiated at the lowest dose.Pulmonary arterial hypertension: The recommended dose of Adegra 50 mg is 20 mg three times a day and should be taken approximately 4-6 hours apart, with or without food.Administration of Adegra 50 mgSildenafil?may takes longer time to work if you take it with a heavy meal.Interaction of Adegra 50 mgSildenafil metabolism is principally mediated by the cytochrome P450 (CYP) isoforms 3A4 (major route) and 2C9 (minor route). Therefore, inhibitors of these isoenzymes 15 may reduce Sildenafil clearance and inducers of these isoenzymes may increase Sildenafil clearance. Cimetidine (800 mg), a nonspecific CYP inhibitor, caused a 56% increase in plasma Sildenafil concentrations when coadministered with Sildenafil (50 mg) to healthy volunteers. When a single 100 mg dose of Sildenafil was administered with erythromycin, a specific CYP3A4 inhibitor, at steady state (500 mg bid for 5 days), there was a 182% increase in Sildenafil systemic exposure (AUC). In addition, in a study performed in healthy male volunteers, co-administration of the HIV protease inhibitor saquinavir, also a CYP3A4 inhibitor, at steady state (1200 mg tid) with Sildenafil (100 mg single dose) resulted in a 140% increase in Sildenafil Cmax and a 210% increase in Sildenafil AUC. Sildenafil had no effect on saquinavir pharmacokinetics. Stronger CYP3A4 inhibitors such as ketoconazole or itraconazole would be expected to have still greater effects, and population data from patients in clinical trials did indicate a reduction in Sildenafil clearance when it was coadministered with CYP3A4 inhibitors (such as ketoconazole, erythromycin, or cimetidine). In another study in healthy male volunteers, coadministration with the HIV protease inhibitor ritonavir, which is a highly potent P450 inhibitor, at steady state (500 mg bid) with Sildenafil (100 mg single dose) resulted in a 300% (4-fold) increase in Sildenafil Cmax and a 1000% (11-fold) increase in Sildenafil plasma AUC. At 24 hours the plasma levels of Sildenafil were still approximately 200 ng/mL, compared to approximately 5 ng/mL when Sildenafil was dosed alone. This is consistent with ritonavir's marked effects on a broad range of P450 substrates. Sildenafil had no effect on ritonavir pharmacokinetics. Although the interaction between other protease inhibitors and Sildenafil has not been studied, their concomitant use is expected to increase Sildenafil levels. In a study of healthy male volunteers, co-administration of Sildenafil at steady state (80 mg t.i.d.) with endothelin receptor antagonist bosentan (a moderate inducer of CYP3A4, CYP2C9 and possibly of cytochrome P450 2C19) at steady state (125 mg b.i.d.) resulted in a 63% decrease of Sildenafil AUC and a 55% decrease in Sildenafil Cmax. Concomitant administration of strong CYP3A4 inducers, such as rifampin, is expected to cause greater decreases in plasma levels of Sildenafil. Single doses of antacid (magnesium hydroxide/aluminum hydroxide) did not affect the bioavailability of Sildenafil. Pharmacokinetic data from patients in clinical trials showed no effect on Sildenafil pharmacokinetics of CYP2C9 inhibitors (such as tolbutamide, warfarin), CYP2D6 inhibitors (such as selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazide and related diuretics, ACE inhibitors, and calcium channel blockers. The AUC of the active metabolite, N-desmethyl Sildenafil, was increased 62% by loop and potassium-sparing diuretics and 102% by 16 nonspecific beta-blockers. These effects on the metabolite are not expected to be of clinical consequence.ContraindicationsSildenafil is contraindicated in patient with hypersensitivity to any component of this medication. Sildenafil potentiates the hypotensive effects of nitrates, so it is contraindicated in patients who are using organic nitrates, either regularly or intermittently.Side Effects of Adegra 50 mgBody as a whole: face edema, photosensitivity reaction, shock, asthenia, pain, chills, accidental fall, abdominal pain, allergic reaction, chest pain, accidental injury.Cardiovascular: angina pectoris, AV block, migraine, syncope, tachycardia, palpitation,hypotension, postural hypotension, myocardial ischemia, cerebral thrombosis, cardiac arrest, heart failure, abnormal electrocardiogram, cardiomyopathy.Digestive: vomiting, glossitis, colitis, dysphagia, gastritis, gastroenteritis, esophagitis, stomatitis, dry mouth, liver function tests abnormal, rectal hemorrhage, gingivitis.Hemic and Lymphatic: anemia and leukopenia.Metabolic and Nutritional: thirst, edema, gout, unstable diabetes, hyperglycemia, peripheral edema, hyperuricemia, hypoglycemic reaction, hypernatremia.Musculoskeletal: arthritis, arthrosis, myalgia, tendon rupture, tenosynovitis, bone pain,myasthenia, synovitis.Nervous: ataxia, hypertonia, neuralgia, neuropathy, paresthesia, tremor, vertigo, depression, insomnia, somnolence, abnormal dreams, reflexes decreased, hyperesthesia.Respiratory: asthma, dyspnea, laryngitis, pharyngitis, sinusitis, bronchitis, sputum increased, cough increased.Skin and Appendages: urticaria, herpes simplex, pruritus, sweating, skin ulcer, contact dermatitis, exfoliative dermatitis.Special Senses: sudden decrease or loss of hearing, mydriasis, conjunctivitis, photophobia, tinnitus, eye pain, ear pain, eye hemorrhage, cataract, dry eyes.Urogenital: cystitis, nocturia, urinary frequency, breast enlargement, urinary incontinence, abnormal ejaculation, genital edema and anorgasmia.Cardiovascular and cerebrovascular: Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of Sildenafil. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of Sildenafil without sexual activity. Others were reported to have occurred hours to days after the use of Sildenafil and sexual activity. It is not possible to determine whether these events are related directly to Sildenafil, to sexual activity, to the patient's underlying 23 cardiovascular disease, to a combination of these factors, or to other factors.Nervous: seizure, seizure recurrence, anxiety, and transient global amnesia.Urogenital: prolonged erection, priapism and hematuria.Special Senses: diplopia, temporary vision loss/decreased vision, ocular redness or bloodshot appearance, ocular burning, ocular swelling/pressure, increased intraocular pressure, retinal vascular disease or bleeding, vitreous detachment/traction, paramacular edema and epistaxis.Pregnancy & LactationPregnancy category B. There are no adequate and well-controlled studies of Sildenafil in pregnant women. Sildenafil is not indicated for use by women. In animal study shows that Sildenafil has no evidence of teratogenicity or embryotoxicity.Precautions & WarningsGeneral: The evaluation of erectile dysfunction should include a determination of potential underlying causes and the identification of appropriate treatment following a complete medical assessment.Before prescribing Sildenafil, it is important to note the following: Caution is advised when Phosphodiesterase Type 5 (PDE5) inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including Sildenafil, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (e.g. dizziness, lightheadedness, fainting).Consideration should be given to the following: Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors. In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest dose. In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. A stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor. Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs. Sildenafil has systemic vasodilatory properties and may augment the blood pressure-lowering effect of other anti-hypertensive medications. Patients on multiple antihypertensive medications were included in the pivotal clinical trials for Sildenafil. In a separate drug interaction study, when amlodipine, 5 mg or 10 mg, and Sildenafil, 100 mg were orally administered concomitantly to hypertensive patients mean additional blood pressure reduction of 8 mmHg systolic and 7 mmHg diastolic were noted. The safety of Sildenafil is unknown in patients with bleeding disorders and patients with active peptic ulceration. Sildenafil should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie's disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia). The safety and efficacy of combinations of Sildenafil with other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended. There is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including Sildenafil, should not be generally used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status. Sildenafil has systemic vasodilatory properties that resulted in transient decreases in supine blood pressure in healthy volunteers (mean maximum decrease of 8.4/5.5 mmHg). While this normally would be expected to be of little consequence in most patients, prior to prescribing Sildenafil, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects, especially in combination with sexual activity.Patients with the following underlying conditions can be particularly sensitive to the actions of vasodilators including Sildenafil - those with left ventricular outflow obstruction (e.g. aortic stenosis, idiopathic hypertrophic subaortic stenosis) and those with severely impaired autonomic control of blood pressure.There is no controlled clinical data on the safety or efficacy of Sildenafil in the following groups; if prescribed, this should be done with caution. Patients who have suffered a myocardial infarction, stroke, or life-threatening arrhythmia within the last 6 months; Patients with resting hypotension (BP 170/110); Patients with cardiac failure or coronary artery disease causing unstable angina; Patients with retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases); Patients with sickle cell or related anemias. Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of Sildenafil. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency could result. If Sildenafil is prescribed to patients taking ritonavir, caution should be used. Data from subjects exposed to high systemic levels of Sildenafil are limited. Visual disturbances occurred more commonly at higher levels of Sildenafil exposure. Decreased blood pressure, syncope, and prolonged erection were reported in some healthy volunteers exposed to high doses of Sildenafil (200-800 mg). To decrease the chance of adverse events in patients taking ritonavir, a decrease in Sildenafil dosage is recommended.Overdose Effects of Adegra 50 mgIn studies with healthy volunteers of single doses up to 800 mg, adverse events were similar to those seen at lower doses but incidence rates and severities were increased. 24 In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance as Sildenafil is highly bound to plasma proteins and it is not eliminated in the urine.Storage ConditionsKeep in a dry place, away from light and heat. Keep out of the reach of children.Drug ClassesDrugs for Erectile DysfunctionMode Of ActionMechanism of Action: The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the enzyme guanylate cyclase, which results in increased levels of cyclic guanosine monophosphate (cGMP), producing smooth muscle relaxation in the corpus cavernosum and allowing inflow of blood. Sildenafil has no direct relaxant effect on isolated human corpus cavernosum, but enhances the effect of nitric oxide (NO) by inhibiting phosphodiesterase type 5 (PDE5), which is responsible for degradation of cGMP in the corpus cavernosum. When sexual stimulation causes local release of NO, inhibition of PDE5 by Sildenafil causes increased levels of cGMP in the corpus cavernosum, resulting in smooth muscle relaxation and inflow of blood to the corpus cavernosum. Sildenafil at recommended doses has no effect in the absence of sexual stimulation.Pharmacokinetics and Metabolism: Sildenafil is rapidly absorbed after oral administration, with a mean absolute bioavailability of 41% (range 25-63%). It is eliminated predominantly by hepatic metabolism (mainly cytochrome P450 3A4) and is converted to an active metabolite with properties similar to the parent, Sildenafil. Both Sildenafil and the metabolite have terminal half lives of about 4 hours.Absorption and Distribution: Sildenafil is rapidly absorbed. Maximum observed plasma concentrations are reached within 30 to 120 minutes (median 60 minutes) of oral dosing in the fasted state. When Sildenafil is taken with a high fat meal, the rate of absorption is reduced.Metabolism and Excretion: Sildenafil is cleared by hepatic microsomal isoenzymes. After either oral or intravenous administration, Sildenafil is excreted as metabolites predominantly in the feces (approximately 80% of administered oral dose) and to a lesser extent in the urine (approximately 13% of the administered oral dose).Pharmacokinetics in Special Populations: Geriatrics: Healthy elderly volunteers (65 years or over) had a reduced clearance of Sildenafil, resulting in approximately 84% and 107% higher plasma AUC values of Sildenafil compared to those seen in healthy younger volunteers.PregnancyPregnancy category B. There are no adequate and well-controlled studies of Sildenafil in pregnant women. Sildenafil is not indicated for use by women. In animal study shows that Sildenafil has no evidence of teratogenicity or embryotoxicity.Sku: 1736099708-2166
Adegra50 mg
₦6,619.80Original price was: ₦6,619.80.₦5,957.60Current price is: ₦5,957.60.₦6,619.80Original price was: ₦6,619.80.₦5,957.60Current price is: ₦5,957.60. Add to basket Quick View -
SaleAdelax 0.5 mg+10 mgAdelax 0.5 mg+10 mg tablet is indicated in- Anxiety Depression Apathy Psychogenic depression. Depressive neurosses. Masked depression. Psychosomatic affections accompanied by anxiety and apathy. Menopausal depressions. Dysphoria and depression in alcoholics and drug addicts.Theropeutic ClassCombined anxiolytics & anti-depressant drugsPharmacologyMaximum serum concentration is reached in about 4 hours after oral administration of Adelax 0.5 mg+10 mg. The half-life of Flupentixol is about 35 hours and that of Melitracen is about 19 hours. The combination of Flupentixol and Melitracen does not seem to influence the pharmacokinetic properties of the individual compounds. Flupenthixol primarily acts as a neuroleptic drug. The antipsychotic effect of Flupenthixol is achieved by mixed blockade of dopamine D1 and D2 receptors. In the mesolimbic dopamine system of the brain, this accounts for the antipsychotic action of this drug. Flupenthixol has other effects on CNS. In the chemoreceptor trigger zone, the dopamine blockade accounts for the antiemetic effect of the drug.Melitracen is a tricyclic antidepressant. It blocks the neuronal re-uptake of both serotonin and nor-epinephrine in the central nervous system, there by minimizing the symptoms of depression.Dosage & Administration of Adelax 0.5 mg+10 mgAdults: Usually 2 tablets daily, in morning and afternoon. In severe cases, the morning dose may be increased to 2 tablets.Elderly patients: 1 tablet in the morning. Maintenance dose: Usually 1 tablet in the morning or as directed by the physician.Dosage of Adelax 0.5 mg+10 mgAdults: Usually 2 tablets orally daily in the morning and noon. In severe cases, the morning dose may be increased to 2 tablets.Elderly patients: 1 tablet in the morning. Maintenance dose: Usually 1 tablet orally in the morning. In cases of insomnia or severe restlessness, additional treatment with a sedative in the acute phase is recommended.Interaction of Adelax 0.5 mg+10 mgThis tablet may enhance the response to alcohol, barbiturates and other CNS depressants. Simultaneous administration of MAO-inhibitors may cause hypertensive crises. Neuroleptics and thymoleptics reduce the antihypertensive effect of guanethidine and similar acting compounds and thymoleptics enhance the effects of adrenaline and noradrenaline.ContraindicationsThe immediate recovery phase after myocardial infarction. Defects in bundle-branch conduction. Untreated narrow-angle glaucoma. Acute alcohol, barbiturate and opiate intoxications. This tablet should not be given to patients who have received an MAO-inhibitor within two weeks. Not recommended for excitable or overactive patients since its activating effect may lead to exaggeration of these characteristics.Side Effects of Adelax 0.5 mg+10 mgIn the recommended doses side effects are rare. These could be transient restlessness and insomnia.Pregnancy & LactationThe safety of this drug has not been established in pregnancy and lactation.Precautions & WarningsIf previously the patient has been treated with tranquillizers with sedative effect these should be withdrawn gradually.Overdose Effects of Adelax 0.5 mg+10 mgIn cases of overdosage the symptoms of intoxications by melitracen, especially of anticholinergic nature, dominate. More rarely extrapyramidal symptoms due to flupentixol occur. Symptomatic and Supportive. Gastric lavage should be carried out as soon as possible and activated charcoal may be administered. Measures aimed at supporting the respiratory and cardiovascular systems should be instituted. Epinephrine (adrenaline) must not be used for such patients. Convulsions may be treated with diazepam and extrapyramidal symptoms with biperiden.Storage ConditionsStore at a temperature not exceeding 30?C in a dry place. Protect from light. Keep out of reach of children.Drug ClassesCombined anxiolytics & anti-depressant drugsMode Of ActionThis consists of two well known and well proven compounds: flupentixol-a neuroleptic with anxiolytic and antidepressant properties of its own when given in small doses, and melitracen-a bipolar thymoleptic with activating properties in low doses. In combination the compounds render a preparation with antidepressant, anxiolytic and activating properties. Maximal serum concentration is reached in about 4 hours after oral administration of flupentixol and in about 4 hours after oral administration of melitracen. The biological half-life of flupentixol is about 35 hours and that of melitracen is about 19 hours. The combination of Adelax 0.5 mg+10 mg does not seem to influence the pharmacokinetic properties of the individual compounds.PregnancyThis tablet should preferably not be given during pregnancy and lactation.Sku: 1736104875-3686
Adelax0.5 mg+10 mg
₦2,761.00Original price was: ₦2,761.00.₦2,484.90Current price is: ₦2,484.90.₦2,761.00Original price was: ₦2,761.00.₦2,484.90Current price is: ₦2,484.90. Add to basket Quick View -
SaleAdempa-M 5 mg+500 mgThis tablet is indicated for the treatment of adults with type 2 diabetes mellitus as an adjunct to diet and exercise: In patients insufficiently controlled on their maximally tolerated dose of Metformin alone In combination with other medicinal products for the treatment of diabetes, in patients insufficiently controlled with Metformin and these medicinal products ... Read moreThis tablet is indicated for the treatment of adults with type 2 diabetes mellitus as an adjunct to diet and exercise: In patients insufficiently controlled on their maximally tolerated dose of Metformin alone In combination with other medicinal products for the treatment of diabetes, in patients insufficiently controlled with Metformin and these medicinal products In patients already being treated with the combination of Empagliflozin and Metformin as separate tablets. Theropeutic ClassCombination Oral hypoglycemic preparationsPharmacologyEmpagliflozin is an inhibitor of Sodium-Glucose Co-Transporter 2 (SGLT2). SGLT2 is the predominant transporter, responsible for reabsorption of glucose from the kidney back into the circulation. By inhibiting SGLT2, Empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose and thereby increases urinary glucose excretion.Metformin Hydrochloride is a biguanide type oral antihyperglycemic drug, used in the management of type 2 diabetes. It lowers both basal and postprandial plasma glucose. It does not produce hypoglycemia. Metformin Hydrochloride decreases hepatic glucose production, decreases intestinal absorption of glucose and improves insulin sensitivity by an increase in peripheral glucose uptake and utilization.Dosage & Administration of Adempa-M 5 mg+500 mgThe dosage should be individualized based on effectiveness and tolerability. Take this combination twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal side effects due to Metformin Hydrochloride. Maximum recommended daily dose of Metformin Hydrochloride is 2000 mg and Empagliflozin is 25 mg.Recommended individualized starting dose: In patients on Metformin Hydrochloride, switch to this combination containing Empagliflozin 5 mg with a similar total daily dose of Metformin Hydrochloride. In patients on Empagliflozin, switch to this combination containing Metformin Hydrochloride 500 mg with a similar total daily dose of Empagliflozin. In patients already treated with Empagliflozin and Metformin Hydrochloride separately switch to this combination containing the same total daily doses of each component. In patients with volume depletion not previously treated with Empagliflozin, correct this condition before initiating this combination. Renal impaired patient: Assess renal function before initiating this combination. In patients with an eGFR below 45 mL/min/1.73 m2 is contraindicated.Dosage of Adempa-M 5 mg+500 mgThe dosage should be individualized based on effectiveness and tolerability. Take this combination twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal side effects due to Metformin Hydrochloride. Maximum recommended daily dose of Metformin Hydrochloride is 2000 mg and Empagliflozin is 25 mg.Recommended individualized starting dose: In patients on Metformin Hydrochloride, switch to this combination containing Empagliflozin 5 mg with a similar total daily dose of Metformin Hydrochloride. In patients on Empagliflozin, switch to this combination containing Metformin Hydrochloride 500 mg with a similar total daily dose of Empagliflozin. In patients already treated with Empagliflozin and Metformin Hydrochloride separately switch to this combination containing the same total daily doses of each component. In patients with volume depletion not previously treated with Empagliflozin, correct this condition before initiating this combination. Renal impaired patient: Assess renal function before initiating this combination. In patients with an eGFR below 45 mL/min/1.73 m2 is contraindicated.Pediatric patients under 18 years of age: Safety and effectiveness in pediatric patients under 18 years of age have not been established.Interaction of Adempa-M 5 mg+500 mgDiuretics: Co-administration of Empagliflozin with diuretics resulted in increased urine volume and frequency of voids, which might enhance the potential for volume depletion.Insulin or Insulin Secretagogues: Co-administration of Empagliflozin with insulin or insulin secretagogues increases the risk for hypoglycemia.Positive Urine Glucose Test: Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.Drugs that Reduce Metformin Clearance: Drugs that reduce Metformin clearance (such as ranolazine, vandetanib, dolutegravir, and cimetidine) may increase the accumulation of Metformin.Carbonic Anhydrase Inhibitors: Carbonic anhydrase inhibitors may increase risk of lactic acidosis.Drugs Affecting Glycemic Control: Thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid produce hoperglycemia. When such drugs are administered to a patient receiving Empagliflozin and Metformin combination, the patient should be closely observed to maintain adequate glycemic control. When such drugs are withdrawn from a patient receiving Empagliflozin and Metformin combination, the patient should be observed closely for hypoglycemia.Alcohol: Alcohol can potentiate the effect of Metformin on lactate metabolism. Warn patients against excessive alcohol intake.Contraindications Hypersensitivity to Empagliflozin and Metformin Any type of acute metabolic acidosis (such as lactic acidosis, diabetic ketoacidosis) Diabetic pre-coma Severe renal failure (GFR 5%) are diarrhea, nausea/vomiting, flatulence, abdominal discomfort, indigestion, asthenia, and headache. The following important adverse reactions are given below: Very common: Hypoglycemia (when used with sulphonylurea or insulin), Gastrointestinal symptoms Common: Vaginal moniliasis, vulvovaginitis, balanitis and other genital infection. Urinary tract infection (including pyelonephritis and urosepsis), thirst, taste disturbance, pruritus (generalised), rash, Increased urination, serum lipids increased Uncommon: Volume depletion, urticaria, dysuria, blood creatinine increased/Glomerular filtration rate decreased, Haematocrit increased Rare: Diabetic ketoacidosis. Pregnancy & LactationAdvise females of the potential risk to a fetus especially during the second and third trimesters. This is not recommended when breastfeeding.Precautions & WarningsLactic Acidosis: Postmarketing cases of Metformin Hydrochloride-associated lactic acidosis. If lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of this combination. Hypotension: Before initiating this combination assess and correct volume status in patients with renal impairment, the elderly, in patients with low systolic blood pressure, and in patients on diuretics. Monitor for signs and symptoms of hypotension after initiating therapy and increase monitoring in clinical situations where volume contraction is expected. Ketoacidosis: Before initiating this combination assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level. If suspected, discontinue this combination, evaluate and treat promptly. Acute kidney injury & impairment in renal function: Consider temporarily discontinuing this combination in settings of reduced oral intake or fluid losses. If acute kidney injury occurs, discontinue this combination promptly and institute treatment. Urosepsis, Pyelonephritis, Fournier?s gangrene & Genital mycotic infections: Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated. Hypoglycemia: Consider lowering the dose of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating this combination. Vitamin B12 Deficiency: Metformin Hydrochloride may lower vitamin B12 levels. Monitor hematologic parameters annually. Increased LDL-C: Monitor and treat as appropriate. Macrovascular Outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with this combination.Overdose Effects of Adempa-M 5 mg+500 mgIn controlled clinical studies single doses of up to 800 mg Empagliflozin (equivalent to 32-times the highest recommended daily dose) in healthy volunteers and multiple daily doses of up to 100 mg Empagliflozin (equivalent to 4-times the highest recommended daily dose) in patients with type 2 diabetes did not show any toxicity. Hypoglycaemia has not been seen with Metformin doses of up to 85 g, although lactic acidosis has occurred in such circumstances. Lactic acidosis is a medical emergency and must be treated in hospital. In the event of an overdose, treatment should be initiated as appropriate to the patient's clinical status. The most effective method to remove lactate and Metformin is haemodialysis. The removal of Empagliflozin by haemodialysis has not been studiedStorage ConditionsKeep below 30?C temperature, protected from light & moisture. Keep out of the reach of children.Use In Special PopulationsSafety and effectiveness in pediatric patients under 18 years of age have not been established.Drug ClassesCombination Oral hypoglycemic preparationsMode Of ActionEmpagliflozin is an inhibitor of Sodium-Glucose Co-Transporter 2 (SGLT2). SGLT2 is the predominant transporter, responsible for reabsorption of glucose from the kidney back into the circulation. By inhibiting SGLT2, Empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose and thereby increases urinary glucose excretion.Metformin Hydrochloride is a biguanide type oral antihyperglycemic drug, used in the management of type 2 diabetes. It lowers both basal and postprandial plasma glucose. It does not produce hypoglycemia. Metformin Hydrochloride decreases hepatic glucose production, decreases intestinal absorption of glucose and improves insulin sensitivity by an increase in peripheral glucose uptake and utilization.PregnancyAdvise females of the potential risk to a fetus especially during the second and third trimesters. This is not recommended when breastfeeding.Sku: 1736097050-1389
Adempa-M5 mg+500 mg
₦11,000.00Original price was: ₦11,000.00.₦9,900.00Current price is: ₦9,900.00.₦11,000.00Original price was: ₦11,000.00.₦9,900.00Current price is: ₦9,900.00. Add to basket Quick View -
SaleAdempa 10 mgAdempa 10 mg is indicated in: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. To reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and established cardiovascular disease. Theropeutic ClassSodium-glucose Cotransporter-2 (SGLT2) InhibitorsPharmacologyAdempa 10 mg is a sodium glucose co-transporter-2 (SGLT-2) inhibitor. SGLT2 co-transporters are responsible for reabsorption of glucose from the glomerular filtrate in the kidney. The glucuretic effect resulting from SGLT2 inhibition reduces renal absorption and lowers the renal threshold for glucose, resulting in increased glucose excretion. Additionally, it contributes to reduced hyperglycaemia, assists weight loss, and reduces blood pressure.Dosage & Administration of Adempa 10 mgThe recommended dose of Adempa 10 mg is 10 mg once daily, taken in the morning, with or without food. In patients tolerating Adempa 10 mg, the dose may be increased to 25 mg once daily. In patients with volume depletion, correcting this condition prior to initiation of Adempa 10 mg is recommended.Dosage of Adempa 10 mgThe recommended dose of Adempa 10 mg is 10 mg once daily, taken in the morning, with or without food. In patients tolerating Adempa 10 mg, the dose may be increased to 25 mg once daily. In patients with volume depletion, correcting this condition prior to initiation of Adempa 10 mg is recommended.Interaction of Adempa 10 mgDiuretics: Co-administration of Adempa 10 mg with diuretics resulted in increased urine volume.?Insulin or Insulin Secretagogues: Co-administration of Adempa 10 mg with insulin or insulin secretagogues increases the risk for hypoglycemia.?Positive Urine Glucose Test: Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.?Interference with 1,5-anhydroglucitol (1,5-AG) Assay: Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.ContraindicationsAdempa 10 mg is contraindicated in patients with history of serious hypersensitivity reaction to Adempa 10 mg or any of its ingredients, severe renal impairment, end-stage renal disease, or dialysis.Side Effects of Adempa 10 mgThe most common adverse reactions associated with Adempa 10 mg are urinary tract infections and female genital mycotic infections. Others common side effects includes dehydration, hypotension, weakness, dizziness and increased thirstiness.Pregnancy & LactationThere are no adequate and well-controlled studies of Adempa 10 mg in pregnant women. Adempa 10 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if Adempa 10 mg is excreted in human milk. It is not recommended when breastfeeding.Precautions & WarningsAssessment of renal function is recommended prior to initiation of Adempa 10 mg and periodically thereafter. Adempa 10 mg should not initiated in patients with an eGFR less than 45 ml/min/1.73 m2. No dose adjustment is needed in patients with an eGFR greater than or equal to 45 ml/min/1.73 m2.Overdose Effects of Adempa 10 mgIn the event of an overdose with Adempa 10 mg the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, perform clinical monitoring, and institute supportive treatment) should be employed. Removal of Adempa 10 mg by hemodialysis has not been studied.Storage ConditionsKeep in a cool & dry place (below 30? C), protected from light & moisture. Keep out of the reach of children.Drug ClassesSodium-glucose Cotransporter-2 (SGLT2) InhibitorsMode Of ActionAdempa 10 mg is a sodium glucose co-transporter-2 (SGLT-2) inhibitor. SGLT2 co-transporters are responsible for reabsorption of glucose from the glomerular filtrate in the kidney. The glucuretic effect resulting from SGLT2 inhibition reduces renal absorption and lowers the renal threshold for glucose, resulting in increased glucose excretion. Additionally, it contributes to reduced hyperglycaemia, assists weight loss, and reduces blood pressure.PregnancyThere are no adequate and well-controlled studies of Adempa 10 mg in pregnant women. Adempa 10 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if Adempa 10 mg is excreted in human milk. It is not recommended when breastfeeding.Sku: 1736099583-2130
Adempa10 mg
₦13,750.00Original price was: ₦13,750.00.₦12,375.00Current price is: ₦12,375.00.₦13,750.00Original price was: ₦13,750.00.₦12,375.00Current price is: ₦12,375.00. Add to basket Quick View -
SaleAdempa 25 mgAdempa 25 mg is indicated in: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. To reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and established cardiovascular disease. Theropeutic ClassSodium-glucose Cotransporter-2 (SGLT2) InhibitorsPharmacologyAdempa 25 mg is a sodium glucose co-transporter-2 (SGLT-2) inhibitor. SGLT2 co-transporters are responsible for reabsorption of glucose from the glomerular filtrate in the kidney. The glucuretic effect resulting from SGLT2 inhibition reduces renal absorption and lowers the renal threshold for glucose, resulting in increased glucose excretion. Additionally, it contributes to reduced hyperglycaemia, assists weight loss, and reduces blood pressure.Dosage & Administration of Adempa 25 mgThe recommended dose of Adempa 25 mg is 10 mg once daily, taken in the morning, with or without food. In patients tolerating Adempa 25 mg, the dose may be increased to 25 mg once daily. In patients with volume depletion, correcting this condition prior to initiation of Adempa 25 mg is recommended.Dosage of Adempa 25 mgThe recommended dose of Adempa 25 mg is 10 mg once daily, taken in the morning, with or without food. In patients tolerating Adempa 25 mg, the dose may be increased to 25 mg once daily. In patients with volume depletion, correcting this condition prior to initiation of Adempa 25 mg is recommended.Interaction of Adempa 25 mgDiuretics: Co-administration of Adempa 25 mg with diuretics resulted in increased urine volume.?Insulin or Insulin Secretagogues: Co-administration of Adempa 25 mg with insulin or insulin secretagogues increases the risk for hypoglycemia.?Positive Urine Glucose Test: Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.?Interference with 1,5-anhydroglucitol (1,5-AG) Assay: Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.ContraindicationsAdempa 25 mg is contraindicated in patients with history of serious hypersensitivity reaction to Adempa 25 mg or any of its ingredients, severe renal impairment, end-stage renal disease, or dialysis.Side Effects of Adempa 25 mgThe most common adverse reactions associated with Adempa 25 mg are urinary tract infections and female genital mycotic infections. Others common side effects includes dehydration, hypotension, weakness, dizziness and increased thirstiness.Pregnancy & LactationThere are no adequate and well-controlled studies of Adempa 25 mg in pregnant women. Adempa 25 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if Adempa 25 mg is excreted in human milk. It is not recommended when breastfeeding.Precautions & WarningsAssessment of renal function is recommended prior to initiation of Adempa 25 mg and periodically thereafter. Adempa 25 mg should not initiated in patients with an eGFR less than 45 ml/min/1.73 m2. No dose adjustment is needed in patients with an eGFR greater than or equal to 45 ml/min/1.73 m2.Overdose Effects of Adempa 25 mgIn the event of an overdose with Adempa 25 mg the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, perform clinical monitoring, and institute supportive treatment) should be employed. Removal of Adempa 25 mg by hemodialysis has not been studied.Storage ConditionsKeep in a cool & dry place (below 30? C), protected from light & moisture. Keep out of the reach of children.Drug ClassesSodium-glucose Cotransporter-2 (SGLT2) InhibitorsMode Of ActionAdempa 25 mg is a sodium glucose co-transporter-2 (SGLT-2) inhibitor. SGLT2 co-transporters are responsible for reabsorption of glucose from the glomerular filtrate in the kidney. The glucuretic effect resulting from SGLT2 inhibition reduces renal absorption and lowers the renal threshold for glucose, resulting in increased glucose excretion. Additionally, it contributes to reduced hyperglycaemia, assists weight loss, and reduces blood pressure.PregnancyThere are no adequate and well-controlled studies of Adempa 25 mg in pregnant women. Adempa 25 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known if Adempa 25 mg is excreted in human milk. It is not recommended when breastfeeding.Sku: 1736099276-2042
Adempa25 mg
₦27,500.00Original price was: ₦27,500.00.₦24,750.00Current price is: ₦24,750.00.₦27,500.00Original price was: ₦27,500.00.₦24,750.00Current price is: ₦24,750.00. Add to basket Quick View -
SaleAdiponil 120 mgAdults: Adiponil 120 mg is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI)>30 kg/m2 and overweight patients (BMI >28 kg/m2 ) with associated risk factors such as type II diabetes, hyperlipidemia ... Read moreAdults: Adiponil 120 mg is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI)>30 kg/m2 and overweight patients (BMI >28 kg/m2 ) with associated risk factors such as type II diabetes, hyperlipidemia and hypertension. Treatment with Adiponil 120 mg should be discontinued after 12 weeks in patients who have not lost at least 5% of their body weight as measured at the start of drug therapy.Adolescents (12 years & older): Obese adolescents should be treated with Adiponil 120 mg only if an adequate reduction of body weight cannot be achieved by means of diet & increased physical activity. Treatment with Adiponil 120 mg should be considered in particular if complications of obesity are present.Theropeutic ClassAppetite suppressant drugs/Anti-obesity drugsPharmacologyAdiponil 120 mg is a reversible inhibitor of gastrointestinal lipase. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipase. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control.Dosage & Administration of Adiponil 120 mgThe recommended dose of Adiponil 120 mg is one 60 mg or 120 mg capsule three times a day with each main meal containing fat (during or up to 1 hour after the meal). The patient should be on a nutritionally balanced, reduced-calorie diet that contains approximately 30% of calories from fat. The daily intake of fat, carbohydrate, and protein should be distributed over three main meals. If a meal is occasionally missed or contains no fat, the dose of Adiponil 120 mg can be omitted. Because Adiponil 120 mg has been shown to reduce the absorption of some fat-soluble vitamins and beta-carotene, patients should be counseled to take a multivitamin containing fat-soluble vitamin to ensure adequate nutrition. The vitamin supplement should be taken at least 2 hours before or after the administration of Adiponil 120 mg, such as at bedtime.Dosage of Adiponil 120 mgThe recommended dose of Adiponil 120 mg is one 120 mg capsule to be taken immediately before, during or up to one hour after each main meal. If a meal is missed or contains no fat the dose of Adiponil 120 mg should be omitted. Doses of Adiponil 120 mg above 120 mg three times daily have not been shown to provide additional benefits. The effect of Adiponil 120 mg results in an increase in fecal fat 24-48 hours after dosing. Upon discontinuation of therapy, fecal fat content usually returns to pretreatment levels within 48-72 hours.The safety & efficacy of Adiponil 120 mg were investigated in clinical studies lasting up to 4 years. The recommended dose of Adiponil 120 mg for adolescents is as same as adults. Special dosage instruction: The tolerability and efficacy of Adiponil 120 mg have not been studied in elderly patients, or patients with hepatic and/ or renal impairments.Interaction of Adiponil 120 mgNo interactions with commonly prescribed medications such as alcohol, digoxin, nifedipine, oral contraceptives, phenytoin, pravastatin, warfarin, or metformin, glibenclamide, fibrates, furosemide, captopril, or atenolol have been observed in studies.ContraindicationsAdiponil 120 mg is contraindicated in patients with chronic malabsorption syndrome, in patients with cholestasis and in patients who are hypersensitive to Adiponil 120 mg or to any of the other ingredients of the capsules.Side Effects of Adiponil 120 mgCommon: Undesirable effects of Adiponil 120 mg are largely gastrointestinal in nature. Common gastrointestinal side effects are oily spotting from the rectum, flatulence, fecal urgency, oily or fatty stool, abdominal discomfort etc.Rare: Influenza, anxiety. headache, fatigue etc may rarely occur in some patients. Rare cases of hypersensitivity have been reported. Main clinical symptoms are pruritus, exanthema, urticaria, angioedema and anaphylaxis.Pregnancy & LactationPregnancy Category X. Adiponil 120 mg is contraindicated during pregnancy, because weight loss offers no potential benefit to a pregnant woman and may result in fetal harm.It is not known whether Adiponil 120 mg is present in human milk or not. Caution should be exercised when Adiponil 120 mg is administered to a nursing woman.Precautions & WarningsOrganic causes of obesity (e.g. hypothyroidism) should be excluded before prescribing Adiponil 120 mg. Adiponil 120 mg and cyclosporine should not be coadministered. Cyclosporine should be taken at least 2 hours before or after Adiponil 120 mg in patients taking both drugs. Cyclosporine level should be measured and frequently monitored.In clinical trial, the decrease in body weight with Adiponil 120 mg therapy was less in type II diabetic patients than in non-diabetic patients. Antidiabetic drug treatment should be closely monitored during Adiponil 120 mg therapy. Because of the improvement in glycemic control, the dose of oral antidiabetics or of insulin may need to be adjusted.Patients should be advised to adhere to the dietary recommendations. The probability of occurrence of gastrointestinal side effects may increase when Adiponil 120 mg is taken with a fatty meal. The daily intake of fat should be distributed between three main meals. Patients should be strongly encouraged to take a multivitamin supplement that contains fat soluble vitamins to ensure adequate nutrition because Adiponil 120 mg has been shown to reduce the absorption of some fat soluble vitamins & beta-carotene. In addition, the levels of vitamin D & beta carotene may be low in obese patients compared with non-obese patients.Overdose Effects of Adiponil 120 mgSingle doses of 800 mg Adiponil 120 mg and multiple doses of up to 400 mg three times a day for 15 days have been studied in normal weight and obese subjects without significant adverse findings.Storage ConditionsStore in cool & dry place below 30?C, protect from light & moisture. Keep out of reach of children.Use In Special PopulationsPediatric use: Safety and effectiveness in pediatric patients below the age of 12 have not been established.Drug ClassesAppetite suppressant drugs/Anti-obesity drugsMode Of ActionAdiponil 120 mg is a potent, specific and long-acting lipase inhibitor. It exerts its therapeutic activity in the lumen of the stomach and upper small intestine by forming a covalent bond with the active serine site of gastric and pancreatic lipases. The inactivated enzyme is thus rendered unable to hydrolyze dietary fats in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides can not be absorbed, a caloric deficit arises which has a positive effect on weight control. Systemic absorption of Adiponil 120 mg is therefore not needed for the activity. At the recommended therapeutic dose of 120 mg three times a day, Adiponil 120 mg inhibit dietary fat absorption by approximately 30%.PregnancyUse in pregnancy & lactation: No clinical data are available on pregnancy exposed to Adiponil 120 mg. As it is not known whether Adiponil 120 mg is excreted in breast milk. Adiponil 120 mg should not be used during breastfeeding.Sku: 1736104922-3699
Adiponil120 mg
₦22,000.00Original price was: ₦22,000.00.₦19,800.00Current price is: ₦19,800.00.₦22,000.00Original price was: ₦22,000.00.₦19,800.00Current price is: ₦19,800.00. Add to basket Quick View -
SaleSku: 1736096583-1255
Adiron30 mg
₦3,630.00Original price was: ₦3,630.00.₦3,267.00Current price is: ₦3,267.00.₦3,630.00Original price was: ₦3,630.00.₦3,267.00Current price is: ₦3,267.00. Add to basket Quick View -
SaleAdlina-EM 25 mg+5 mgThis is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Empagliflozin is indicated to reduce ... Read moreThis is a combination of empagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Empagliflozin is indicated to reduce the risk of cardiovascular death in adults with type 2 diabetes mellitus and established cardiovascular disease.Dosage of Adlina-EM 25 mg+5 mgAssess renal function before initiating and as clinically indicated. The recommended dose of this is 10 mg empagliflozin and 5 mg linagliptin once daily, taken in the morning, with or without food. Dose may be increased to 25 mg empagliflozin and 5 mg linagliptin once daily.Pediatric Patients: The safety and effectiveness in pediatric patients have not been established.Geriatric Patients: Higher incidence of adverse reactions related to volume depletion and reduced renal function.Renal Impairment: Higher incidence of adverse reactions related to reduced renal function.Side Effects of Adlina-EM 25 mg+5 mgThe most common side effects are: urinary tract infection stuffy or runny nose and sore throat upper respiratory tract infection Precautions & WarningsPancreatitis: There have been reports of acute pancreatitis, including fatal pancreatitis. If pancreatitis is suspected, promptly discontinue this tablet.Ketoacidosis: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis, regardless of blood glucose level. If suspected, discontinue this tablet, evaluate and treat promptly. Before initiating this tablet, consider risk factors for ketoacidosis. Patients on this tablet may require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis.Volume Depletion: Before initiating this tablet, assess volume status and renal function in patients with impaired renal function, elderly patients, or patients on loop diuretics. Monitor for signs and symptoms during therapy.Urosepsis and Pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated.Hypoglycemia: Consider lowering the dose of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating this tablet.Necrotizing Fasciitis of the Perineum (Fournier?s Gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment.Genital Mycotic Infections: Monitor and treat as appropriate.Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema, and exfoliative skin conditions) have occurred with empagliflozin and linagliptin. If hypersensitivity reactions occur, discontinue this tablet, treat promptly, and monitor until signs and symptoms resolve.Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue drug if appropriate.Bullous Pemphigoid: There have been reports of bullous pemphigoid requiring hospitalization. Tell patients to report the development of blisters or erosions. If bullous pemphigoid is suspected, discontinue this tablet.Heart Failure: Heart failure has been observed with two other members of the DPP-4 inhibitor class. Consider risks and benefits of this tablet in patients who have known risk factors for heart failure. Monitor for signs and symptoms.Storage ConditionsKeep below 30?C temperature, away from light & moisture. Keep out of the reach of children.Mode Of ActionEmpagliflozin: Sodium-glucose co-transporter 2 (SGLT2) is the predominant transporter responsible for the reabsorption of glucose from the glomerular filtrate back into the circulation. Empagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increasing urinary glucose excretion. ? Linagliptin: Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha cells, resulting in a reduction in hepatic glucose output.PregnancyAdvise females of the potential risk to a fetus, especially during the second and third trimesters. This is not recommended when breastfeeding.Sku: 1736094724-764
Adlina-EM25 mg+5 mg
₦22,000.00Original price was: ₦22,000.00.₦19,800.00Current price is: ₦19,800.00.₦22,000.00Original price was: ₦22,000.00.₦19,800.00Current price is: ₦19,800.00. Add to basket Quick View -
SaleAdlinameg 2.5 mg+500 mgThis is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both Linagliptin and Metformin Hydrochloride is appropriateTheropeutic ClassCombination Oral hypoglycemic preparationsPharmacologyLinagliptin is indicated to improve glycemic control in patients with type 2 diabetes mellitus. Linagliptin is an inhibitor of DPP-4 (dipeptidyl peptidase-4), an enzyme that degrades the incretin hormones GLP-1 (glucagon like peptide-1) and GIP (glucose dependent insulinotropic polypeptide). Thus, Linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin from pancreatic beta (?) cells in a glucose-dependent manner and decreasing the secretion of glucagon from pancreatic alpha (?) cells in the circulation.Metformin Hydrochloride is a biguanide type oral antihyperglycemic drug used in the management of type 2 diabetes. It lowers both basal and postprandial plasma glucose. Its mechanism of action is different from those of sulfonylureas and it does not produce hypoglycemia. Metformin Hydrochloride decreases hepatic glucose production, decreases intestinal absorption of glucose and improves insulin sensitivity by an increase in peripheral glucose uptake and utilization.Dosage & Administration of Adlinameg 2.5 mg+500 mgLinagliptin & Metformin immediate release tablet: The dosage of Linagliptin & Metformin should be individualized on the basis of both effectiveness and tolerability. Maximum recommended dose of 2.5 mg Linagliptin and 1000 mg Metformin Hydrochloride twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with Metformin Hydrochloride use.Recommended starting dose: In patients currently not treated with Metformin Hydrochloride, initiate treatment with 2.5 mg Linagliptin and 500 mg Metformin Hydrochloride twice daily. In patients already treated with Metformin Hydrochloride, start with 2.5 mg Linagliptin and the current dose of Metformin Hydrochloride twice daily. Patients already treated with Linagliptin and Metformin Hydrochloride, individual components may be switched to this combination containing the same doses of each component.Linagliptin & Metformin extend release tablet: The dosage of this combination should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended total daily dose of Linagliptin 5 mg and Metformin Hydrochloride 2000 mg. this combination should be given once daily with a meal.Recommended starting dose: In patients currently not treated with metformin, initiate this combination treatment with 5 mg Linagliptin/1000 mg Metformin Hydrochloride extended-release once daily with a meal.In patients already treated with Metformin, start this combination with 5 mg of Linagliptin total daily dose and a similar total daily dose of Metformin once daily with a meal.In patients already treated with Linagliptin & Metformin immediate release tablet, switch to extend release tablet containing 5 mg of Linagliptin total daily dose and a similar total daily dose of Metformin once daily with a meal.5 mg Linagliptin & 1000 mg Metformin Hydrochloride extended-release tablet should be taken as a single tablet once daily. Patients using 2.5 mg Linagliptin & 1000 mg Metformin extended release tablets should take two tablets together once daily.Dosage of Adlinameg 2.5 mg+500 mgLinagliptin & Metformin immediate release tablet: The dosage of Linagliptin & Metformin should be individualized on the basis of both effectiveness and tolerability. Maximum recommended dose of 2.5 mg Linagliptin and 1000 mg Metformin Hydrochloride twice daily with meals. Dose escalation should be gradual to reduce the gastrointestinal (GI) side effects associated with Metformin Hydrochloride use.Recommended starting dose: In patients currently not treated with Metformin Hydrochloride, initiate treatment with 2.5 mg Linagliptin and 500 mg Metformin Hydrochloride twice daily. In patients already treated with Metformin Hydrochloride, start with 2.5 mg Linagliptin and the current dose of Metformin Hydrochloride twice daily. Patients already treated with Linagliptin and Metformin Hydrochloride, individual components may be switched to this combination containing the same doses of each component.Linagliptin & Metformin extend release tablet: The dosage of this combination should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended total daily dose of Linagliptin 5 mg and Metformin Hydrochloride 2000 mg. this combination should be given once daily with a meal.Recommended starting dose: In patients currently not treated with metformin, initiate this combination treatment with 5 mg Linagliptin/1000 mg Metformin Hydrochloride extended-release once daily with a meal.In patients already treated with Metformin, start this combination with 5 mg of Linagliptin total daily dose and a similar total daily dose of Metformin once daily with a meal.In patients already treated with Linagliptin & Metformin immediate release tablet, switch to extend release tablet containing 5 mg of Linagliptin total daily dose and a similar total daily dose of Metformin once daily with a meal.5 mg Linagliptin & 1000 mg Metformin Hydrochloride extended-release tablet should be taken as a single tablet once daily. Patients using 2.5 mg Linagliptin & 1000 mg Metformin extended release tablets should take two tablets together once daily.Interaction of Adlinameg 2.5 mg+500 mgCationic drugs (amiloride, digoxin, morphine, ranitidine, trimethoprim etc.): May reduce metformin elimination. P-glycoprotien/CYP3A4 inducer (i.e. rifampin): The efficacy of this medicine may be reduced when administered in combination.ContraindicationsAlthough Linagliptin undergoes minimal renal excretion, Metformin Hydrochloride is known to be substantially excreted by the kidney. The risk of Metformin Hydrochloride accumulation and lactic acidosis increases with the degree of renal impairment. Therefore, this combination is contraindicated in patients with renal impairment. It is also contraindicated in acute or chronic metabolic acidosis (diabetic ketoacidosis) and in hypersensitivity to Linagliptin or Metformin Hydrochloride.Side Effects of Adlinameg 2.5 mg+500 mgMost common side effects are nasopharyngitis and diarrhea. Hypoglycemia is more common in patients treated with this combination and sulfonylureas.Pregnancy & LactationThere are no adequate and well-controlled studies in pregnant women with this combination or its individual component; so it should be used during pregnancy only if clearly needed. Caution should also be excercised when it is administered to a lactating mother.Precautions & WarningsIn a patient with lactic acidosis who is taking Metformin, the drug should be discontinued immediately and supportive therapy promptly instituted. There have been postmarketing reports of acute pancreatitis. If pancreatitis is suspected, promptly discontinue Linagliptin & Metformin. Temporarily discontinue Linagliptin & Metformin?in patients undergoing radiologic studies with intravascular administration of iodinated contrast materials or any surgical procedures necessitating restricted intake of food and fluids. Metformin may lower Vitamin B12 levels; so hematologic parameters shoud be monitored annually.Overdose Effects of Adlinameg 2.5 mg+500 mgIn the event of an overdose with this combination the usual supportive measures (i.e. remove unabsorbed material from the gastrointestinal tract, perform clinical monitoring, and institute supportive treatment) should be employed. Removal of Linagliptin by hemodialysis or peritoneal dialysis is unlikely but Metformin Hydrochloride is dialyzable. During controlled clinical trials in healthy subjects, with single doses of up to 600 mg of Linagliptin (equivalent to 120 times the recommended daily dose), there were no dose-related clinical adverse drug reactions. Overdose of Metformin Hydrochloride has occurred in case of ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with Metformin Hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of Metformin Hydrochloride overdose cases.Storage ConditionsKeep in a cool & dry place (below 30?C), protected from light & moisture. Keep out of the reach of children.Drug ClassesCombination Oral hypoglycemic preparationsMode Of ActionLinagliptin is indicated to improve glycemic control in patients with type 2 diabetes mellitus. Linagliptin is an inhibitor of DPP-4 (dipeptidyl peptidase-4), an enzyme that degrades the incretin hormones GLP-1 (glucagon like peptide-1) and GIP (glucose dependent insulinotropic polypeptide). Thus, Linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin from pancreatic beta (?) cells in a glucose-dependent manner and decreasing the secretion of glucagon from pancreatic alpha (?) cells in the circulation.Metformin Hydrochloride is a biguanide type oral antihyperglycemic drug used in the management of type 2 diabetes. It lowers both basal and postprandial plasma glucose. Its mechanism of action is different from those of sulfonylureas and it does not produce hypoglycemia. Metformin Hydrochloride decreases hepatic glucose production, decreases intestinal absorption of glucose and improves insulin sensitivity by an increase in peripheral glucose uptake and utilization.PregnancyThere are no adequate and well-controlled studies in pregnant women with this combination or its individual component; so it should be used during pregnancy only if clearly needed. Caution should also be excercised when it is administered to a lactating mother.Sku: 1736103756-3357
Adlinameg2.5 mg+500 mg
₦6,600.00Original price was: ₦6,600.00.₦5,940.00Current price is: ₦5,940.00.₦6,600.00Original price was: ₦6,600.00.₦5,940.00Current price is: ₦5,940.00. Add to basket Quick View