-
SEDOGEST 300MG TABLET 10?S ₦7,280.75 QTY: 1
-
BUDAMATE 400MG TRANSCAPS ₦4,942.00 QTY: 1
-
Adalat Retard 10mg Tablets, 56 Tablets ₦8,999.00 QTY: 4
-
IMAGE Prevention+ Daily Perfecting Primer SPF 50 ₦122,453.34 QTY: 1
-
Kentofer Capsules 20's ₦135.00 QTY: 2
-
Viktor & Rolf BonBon Eau De Parfum ₦320,103.73 QTY: 1
-
Sebamed Clear Face Care Gel, 50ml ₦31,500.00 QTY: 1
-
2 Way Catheter Size 20 ₦520.00 QTY: 1
-
GLUCOBAY 50MG TAB 10`S ₦3,193.00 QTY: 2
-
CLOTRIN 15GM CREAM ₦1,011.75 QTY: 1
-
Melona-HA Serum 30ml ₦2,336.00 QTY: 1
-
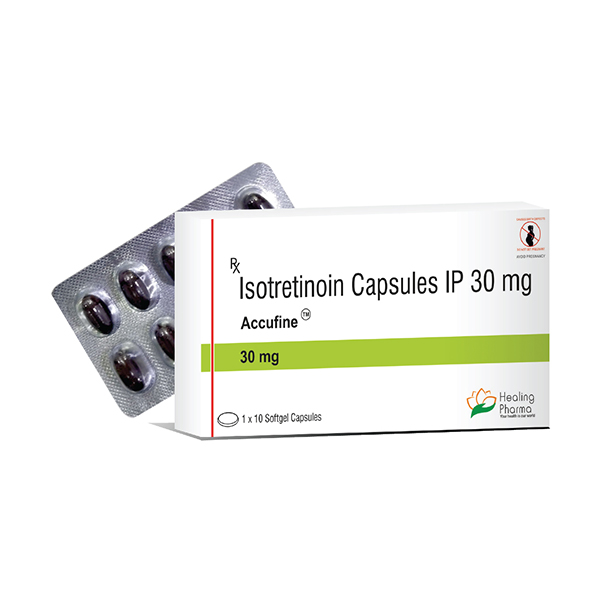
ACCUFINE 30mg Capsule 10?s ₦45,900.00 QTY: 1
-
SIBOFIX 550MG TABLET ₦10,266.00 QTY: 1
-
Sangobion Capsule ₦197.00 QTY: 1
-
Stud 100 Desensitizing Spray For Men ? Lidocaine 9.6% Spray, 12G ₦10,250.00 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
113–128 of 4627 Results
-
SaleAmdocal Plus 5 mg+50 mgAmlodipine & Atenolol is indicated in: Hypertension not controlled by monotherapy Angina pectoris & hypertension co-existing diseases Post MI patients Refractory angina pectoris where nitrate therapy has failed Theropeutic ClassCombined antihypertensive preparationsPharmacologyAmlodipine is a dihydropyridine calcium antagonist that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle; it has a greater effect on vascular muscle than on cardiac muscle. Amlodipine is a peripheral vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. Amlodipine reduces tone, decreases coronary vasoreactivity and lowers cardiac demand by reducing afterload. Atenolol is a cardio selective beta blocker. The cardio selectivity is dose related. Atenolol causes a reduction in blood pressure by lowering cardiac output, decreasing the plasma renin activity and sympathetic outflow from CNS. Atenolol also causes a reduction in myocardial oxygen demand by virtue of its negative inotropic and negative chronotropic effects.Dosage & Administration of Amdocal Plus 5 mg+50 mgThe recommended dosage is one tablet daily of (Amlodipine 5 mg & Atenolol 50 mg) or (Amlodipine 5 mg & Atenolol 25 mg). Depending upon the therapeutic response, titration of the dosage is recommended. In elderly patients, it is advisable to initiate the therapy with ? tablet of fixed dose combination of Amlodipine & Atenolol (i.e. 2.5 mg of Amlodipine & 25 mg Atenolol).Interaction of Amdocal Plus 5 mg+50 mgAmlodipine has been safely administered with thiazide diuretics, beta blockers, alpha blockers, angiotensin converting enzyme inhibitors, long-acting nitrates, sublingual glyceryl trinitrate, non-steroidal anti-inflammatory agents, antibiotics, and oral hypoglycemic agents. In vitro data from studies with human plasma indicate that amlodipine has no effect on protein binding of the drugs tested (Digoxin, Phenytoin, Warfarin, or Indomethacin). Atenolol reduces the clearance of Disopyramide by 20%. Additive negative inotropic effects on the heart may be produced. At doses of 1 gm and above, Ampicillin may reduce Atenolol levels. Beta-blockers may decrease tissue sensitivity to Insulin and inhibit Insulin secretion, e.g. in response to oral antidiabetics. Atenolol has less potential for these actions.ContraindicationsHypersensitivity to either component, sinus bradycardia, second and higher degrees of heart block, cardiogenic shock, hypotension, congestive heart failure, poor left ventricular function.Side Effects of Amdocal Plus 5 mg+50 mgThe combination of Amlodipine and Atenolol is well tolerated. Overall side effects include fatigue, headache, edema, nausea, drowsiness, anxiety and depression.Pregnancy & LactationAtenolol crosses the placenta. So it is contraindicated in pregnancy. It should be avoided during lactation.Precautions & WarningsAtenolol may mask the symptoms of hyperthyroidism. It may also mask the symptoms of hypoglycaemia, as well as enhance the effects of hypoglycaemic agents in patients with diabetes mellitus.Storage ConditionsStore in a cool dry place protected from light. Keep out of reach of children.Sku: 1736107847-4572
Amdocal Plus5 mg+50 mg
₦4,675.00Original price was: ₦4,675.00.₦4,207.50Current price is: ₦4,207.50.₦4,675.00Original price was: ₦4,675.00.₦4,207.50Current price is: ₦4,207.50. Add to basket Quick View -
SaleAmdocal Pro? 2.5 mg+5 mgBisoprolol & Amlodipine combination is indicated for the treatment of hypertension as substitution therapy in patients adequately controlled with the individual products given concurrently at the same dose level as in the combination, but as separate tabletsDosage of Amdocal Pro? 2.5 mg+5 mgOne tablet once daily in patients whose blood pressure is adequately controlled with separately administered monocomponent products of the same doses as the recommended fixed-dose combination.Interaction of Amdocal Pro? 2.5 mg+5 mgCombinations not recommended: Calcium antagonists of the verapamil and diltiazem type, centrally-acting antihypertensive drugs. Combinations to be used with caution: Strong or moderate CYP3A4 inhibitors, CYP3A4 inducers, simvastatin, Tacrolimus, Cyclosporine, class I antiarrhythmic drugs, class III antiarrhythmic drugs, parasympathomimetic drugs, topical beta-blockers (e.g. eye drops), insulin and oral antidiabetic drugs, anesthetic agents, digitalis glycosides, non-steroidal anti-inflammatory drugs (NSAIDs), sympathomimetic agents, antihypertensive agents and other drugs with blood pressure lowering potential. Combinations to be considered: Mefloquine, Rifampicin, Ergotamine derivatives, MAO inhibitors (except MAO-B inhibitor).ContraindicationsAcute heart failure or during episodes of heart failure decompensation, obstruction of the outflow tract of the left ventricle (e.g. high grade aortic stenosis), cardiogenic shock, second or third degree AV block, sick sinus syndrome, sinoatrial block, symptomatic bradycardia or hypotension, severe bronchial asthma, severe forms of peripheral arterial occlusive disease or severe forms of Raynaud?s syndrome, untreated phaeochromocytoma metabolic acidosis, hypersensitivity to bisoprolol, amlodipine, dihydropyridine derivates or to any of the excipients.Side Effects of Amdocal Pro? 2.5 mg+5 mgCommon: Dizziness, headache, somnolence, palpitations, flushing, feeling of coldness or numbness in the extremities, gastrointestinal complaints such as nausea, vomiting, diarrhea, constipation, abdominal pain; edema (e.g. ankle edema), fatigue. Uncommon: Insomnia, mood changes (incl. anxiety), depression, sleep disorders, hypaesthesia, paresthesia, dysgeusia, tremor, visual disturbances (incl. diplopia), tinnitus, AV conduction disturbances, worsening of pre existing heart failure, bradycardia, hypotension, syncope, dyspnea, bronchospasm in patients with bronchial asthma or a history of obstructive airway disease, rhinitis, dyspepsia, dry mouth, alopecia, purpura, skin discoloration, pruritus, exanthema, arthralgia, myalgia, muscular weakness, muscle cramps, back pain, micturition disorder, nocturia, pollakisuria, potency disorders, gynecomastia, asthenia, chest pain, pain, malaise, weight increase, weight decrease. Rare: Allergic reactions mainly affecting the skin, nightmares, hallucinations, confusion, decreased tear secretion, hearing disorders, allergic rhinitis, hepatitis, increased triglycerides, increased liver enzymes (ALAT, ASAT).Precautions & WarningsPatients with heart failure should be treated with caution. An increased risk of a further deterioration of the ventricular pump function cannot be excluded. Since the abrupt withdrawal of bisoprolol may lead to a transitory worsening of the clinical condition, especially in patients with ischemic heart disease, the treatment must not be stopped abruptly. Caution is advised in patients with impaired hepatic function. Beta-blockers should be avoided in patients with obstructive airways diseases unless there are compelling clinical reasons for their use. Due to the bisoprolol component treatment must be used with caution in: bronchospasm (bronchial asthma, chronic obstructive airways disease; concomitant bronchodilating therapy may be recommended); diabetes mellitus showing large fluctuations in blood glucose values, symptoms of hypoglycemia can be masked; strict fasting; ongoing desensitization therapy; first degree AV block; Prinzmetal?s angina; peripheral arterial occlusive disease. Patients with psoriasis or with a history of psoriasis should only be given beta-blockers (e.g. bisoprolol) after a careful balancing of benefits and risks. Symptoms of thyrotoxicosis may be masked. In patients undergoing general anesthesia, the anesthetist must be aware of beta-blockade. If it is thought necessary to withdraw beta blocker therapy before surgery, this should be done gradually and completed about 48 hours before anesthesia.Overdose Effects of Amdocal Pro? 2.5 mg+5 mgMost common signs expected with overdose of a beta-blocker are bradycardia, hypotension, bronchospasm, acute cardiac insufficiency, hypoglycemia. According to available data gross overdose of amlodipine could result in excessive peripheral vasodilation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported. In general, if overdose occurs, discontinuation of treatment and supportive and symptomatic treatment is recommended.Storage ConditionsKeep in a dry place, below 30?C. Protect from light. Keep out of the reach of children.Drug ClassesAnti-hypertensiveMode Of ActionThis consists of Amlodipine and Bisoprolol Fumarate. Amlodipine is a dihydropyridine calcium antagonist that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Amlodipine acts directly on vessels to cause a reduction in peripheral vascular resistance and reduction in blood pressure.Bisoprolol Fumarate is a synthetic, beta1-selective (cardioselective) adrenoceptor blocking agent, lacking intrinsic sympathomimetic and relevant membrane stabilizing activity. It only shows low affinity to the beta2 receptor of the smooth muscles of bronchi and vessels as well as to the beta2-receptors concerned with metabolic regulation. Therefore, bisoprolol is generally not to be expected to influence airway resistance and beta2-mediated metabolic effects. Its beta1-selectivity extends beyond the therapeutic dose range.PregnancyPregnancy and Lactation: Not recommended.Pediatric UsesGeriatric use: The usual doses can be administered to elderly people; however, caution is advised when the dose is increased.Pediatric use: The safety and efficacy of Bisoprolol fumarate/amlodipine in children and adolescents below the age of 18 years have not been established. No data are available.Patients with Liver disease: In case of hepatic impairment elimination of amlodipine may be elongated. Exact dosage recommendations concerning amlodipine have not been established, but the drug should therefore be administered with special caution in these patients. In case of severe hepatic impairment, the daily dose of bisoprolol must not exceed 10 mg.Patients with Kidney disease: No dosage adjustment is required for patients with mild to moderate renal impairment. Amlodipine is not dialyzable. Amlodipine should be administered with particular caution to patients undergoing dialysis. In case of severe renal impairment (creatinine clearance <20 ml/min) the daily dose of bisoprolol must not exceed 10 mgSku: 1736103293-3225
Amdocal Pro?2.5 mg+5 mg
₦3,300.00Original price was: ₦3,300.00.₦2,970.00Current price is: ₦2,970.00.₦3,300.00Original price was: ₦3,300.00.₦2,970.00Current price is: ₦2,970.00. Add to basket Quick View -
SaleAmdocal 10 mgAmdocal 10 mg is a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of Hypertension and Coronary Artery Disease (such as Chronic Stable Angina, Vasospastic Angina and Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction 1.0% are headache, fatigue, nausea, abdominal pain and somnolence.Pregnancy & LactationPregnancy Category C. There are no adequate and well-controlled studies of Amdocal 10 mg in pregnant women. Amdocal 10 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Amdocal 10 mg is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while Amdocal 10 mg is administered.Precautions & WarningsSymptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of Amdocal 10 mg, particularly in patients with severe obstructive coronary artery disease. Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment.Overdose Effects of Amdocal 10 mgIn humans, experience with intentional overdose is limited.Symptoms: Available data suggest that large overdosage could result in excessive peripheral vasodilatation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.Management: Clinically significant hypotension due to Amdocal 10 mg overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output.?A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade. Gastric lavage may be worthwhile in some cases. In healthy volunteers the use of charcoal up to 2 hours after administration of Amdocal 10 mg 10 mg has been shown to reduce the absorption rate of Amdocal 10 mg. Since Amdocal 10 mg is highly protein-bound, dialysis is not likely to be of benefit.Storage Conditionskeep in a dry place away from light and heat. Keep out of the reach of children.Use In Special PopulationsChildren with hypertension from 6 years to 17 years of age: 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients.Children under 6 years old: ?The effect of Amdocal 10 mg on blood pressure in patients less than 6 years of age is not known.Elderly: Amdocal 10 mg used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care.Renal impairment: Changes in Amdocal 10 mg plasma concentrations are not correlated with degree of renal impairment, therefore the normal dosage is recommended. Amdocal 10 mg is not dialysable.Hepatic impairment: Dosage recommendations have not been established in patients with mild to moderate hepatic impairment; therefore dose selection should be cautions and should start at the lower end of the dosing range. The pharmacokinetics of Amdocal 10 mg have not been studied in severe hepatic impairment. Amdocal 10 mg should be initiated at the lowest dose (2.5 mg once daily) and titrated slowly in patients with severe hepatic impairment.Sku: 1736106759-4244
Amdocal10 mg
₦6,600.00Original price was: ₦6,600.00.₦5,940.00Current price is: ₦5,940.00.₦6,600.00Original price was: ₦6,600.00.₦5,940.00Current price is: ₦5,940.00. Add to basket Quick View -
SaleAmdocal 2.5 mgEssential hypertension: Amlodipine is efficacious as monotherapy in the treatment of hypertension. It may be used in combination with other antihypertensive agents.Angina pectoris: Amlodipine is indicated for the treatment of chronic stable angina pectoris ... Read moreEssential hypertension: Amlodipine is efficacious as monotherapy in the treatment of hypertension. It may be used in combination with other antihypertensive agents.Angina pectoris: Amlodipine is indicated for the treatment of chronic stable angina pectoris and is efficacious as monotherapy. It may be used in combination with other antianginal agents.Vasospastic angina: Amlodipine is indicated for the treatment of confirmed or suspected vasospastic angina. It may be used as monotherapy or in combination with other antianginal drugs.Dosage of Amdocal 2.5 mgHypertension: Usual dose is 5 mg once daily. The maximum dose is 10 mg once daily. Elderly patients with hepatic insufficiency may be started on 2.5 mg once daily; this dose may also be used when adding Amlodipine to other antihypertensive therapy.Angina (Chronic stable or Vasospastic): 5 to 10 mg, using the lower dose for elderly and in patients with hepatic insufficiency. Most patients require 10 mg.Administrations: May be taken without regard to meals.Interaction of Amdocal 2.5 mgDrug Interactions- Potentially hazardous interactions: Little or no data are available in patients with markedly impaired cardiac left ventricular function; however, as with other calcium antagonist drugs, the combination of Amlodipine and p-blockers should be avoided in such patients. Other Significant Interactions- Digoxin: Absence of any interaction between Amlodipine and Digoxin in healthy volunteers has been documented in a controlled clinical study. Cimetidine: An unpublished clinical study indicated no interaction between, Amlodipine and Cimetidine in healthy volunteers. Warfarin: An unpublished clinical study in healthy volunteers indicates that Amlodipine did not significantly alter the effect of Warfarin on prothrombin time. Food: Food does not alter the rate or extent of absorption of Amlodipine. ContraindicationsHypersensitivity to dihydropyridine derivatives. Pregnant woman.Side Effects of Amdocal 2.5 mgThe most common adverse effects of amlodipine are associated with vasodilatory action, such as dizziness, flushing, headache, hypotension and peripheral edema. Gastrointestinal disturbances, increased micturition frequency, lethargy, eye pain and mental depression may also occur. A paradoxical increase in ischaemic chest pain may occur at the start of the treatment and in a few patients excessive fall in blood pressure has led to cerebral or myocardial ischaemia or transient blindness. Rashes, fever and abnormalities in liver function due to hypersensitivity reaction of Amlodipine may occur.Precautions & WarningsPrecaution should be taken in patients with hepatic impairment and during pregnancy and breast feeding.Overdose Effects of Amdocal 2.5 mgSymptoms: Available data suggest that large overdosage could result in excessive peripheral vasodilatation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.Management: Clinically significant hypotension due to amlodipine overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output.?A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade. Gastric lavage may be worthwhile in some cases. In healthy volunteers the use of charcoal up to 2 hours after administration of amlodipine 10 mg has been shown to reduce the absorption rate of amlodipine. Since amlodipine is highly protein-bound, dialysis is not likely to be of benefit.Storage ConditionsKeep all medicines out of reach of children. Store in a cool & dry place, protected from light.Drug ClassesCalcium-channel blockersMode Of ActionAmlodipine is a dihydropyridine calcium-channel blocker, with a long duration of action, used for the treatment of hypertension and angina pectoris. Amlodipine influences the myocardial cells, the cells within the specialized conducting system of the heart, and the cells of vascular smooth muscle. Administration of Amlodipine results primarily in vasodilation, with reduced peripheral resistance, blood pressure and afterload, increased coronary blood flow and a reflex increase in coronary heart rate. This in turn results in an increase in myocardial oxygen supply and cardiac output.PregnancyPregnancy Category C. There are no adequate and well-controlled studies of Amlodipine in pregnant women. Amlodipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Amlodipine is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while Amlodipine is administered.Pediatric UsesChildren with hypertension from 6 years to 17 years of age: 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients.Children under 6 years old: ?The effect of amlodipine on blood pressure in patients less than 6 years of age is not known.Elderly: Amlodipine used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care.Renal impairment: Changes in amlodipine plasma concentrations are not correlated with degree of renal impairment, therefore the normal dosage is recommended. Amlodipine is not dialysable.Hepatic impairment: Dosage recommendations have not been established in patients with mild to moderate hepatic impairment; therefore dose selection should be cautions and should start at the lower end of the dosing range. The pharmacokinetics of Amlodipine have not been studied in severe hepatic impairment. Amlodipine should be initiated at the lowest dose (2.5 mg once daily) and titrated slowly in patients with severe hepatic impairment.Sku: 1736097943-1650
Amdocal2.5 mg
₦1,650.00Original price was: ₦1,650.00.₦1,485.00Current price is: ₦1,485.00.₦1,650.00Original price was: ₦1,650.00.₦1,485.00Current price is: ₦1,485.00. Add to basket Quick View -
SaleAmdocal 5 mgAmdocal 5 mg is a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of Hypertension and Coronary Artery Disease (such as Chronic Stable Angina, Vasospastic Angina and Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction 1.0% are headache, fatigue, nausea, abdominal pain and somnolence.Pregnancy & LactationPregnancy Category C. There are no adequate and well-controlled studies of Amdocal 5 mg in pregnant women. Amdocal 5 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Amdocal 5 mg is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while Amdocal 5 mg is administered.Precautions & WarningsSymptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of Amdocal 5 mg, particularly in patients with severe obstructive coronary artery disease. Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment.Overdose Effects of Amdocal 5 mgIn humans, experience with intentional overdose is limited.Symptoms: Available data suggest that large overdosage could result in excessive peripheral vasodilatation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.Management: Clinically significant hypotension due to Amdocal 5 mg overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output.?A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade. Gastric lavage may be worthwhile in some cases. In healthy volunteers the use of charcoal up to 2 hours after administration of Amdocal 5 mg 10 mg has been shown to reduce the absorption rate of Amdocal 5 mg. Since Amdocal 5 mg is highly protein-bound, dialysis is not likely to be of benefit.Storage Conditionskeep in a dry place away from light and heat. Keep out of the reach of children.Use In Special PopulationsChildren with hypertension from 6 years to 17 years of age: 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients.Children under 6 years old: ?The effect of Amdocal 5 mg on blood pressure in patients less than 6 years of age is not known.Elderly: Amdocal 5 mg used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care.Renal impairment: Changes in Amdocal 5 mg plasma concentrations are not correlated with degree of renal impairment, therefore the normal dosage is recommended. Amdocal 5 mg is not dialysable.Hepatic impairment: Dosage recommendations have not been established in patients with mild to moderate hepatic impairment; therefore dose selection should be cautions and should start at the lower end of the dosing range. The pharmacokinetics of Amdocal 5 mg have not been studied in severe hepatic impairment. Amdocal 5 mg should be initiated at the lowest dose (2.5 mg once daily) and titrated slowly in patients with severe hepatic impairment.Frequently Asked Questions Q: What is Amdocal 5mg? Amdocal 5mg is amlodipine besylate. It is a calcium channel blocker for relaxing the blood vessels and lowering blood pressure. Q: What is Amdocal 5mg used for? People take Amdocal 5mg to treat high blood pressure and chest pain or pressure (angina). Q: How does Amdocal 5mg work? Amdocal 5mg relaxes the blood vessels, which helps to lower blood pressure. It blocks the movement of calcium ions into the cells of the heart and blood vessels. Q: What are the side effects of Amdocal 5mg? The common side effects of Amdocal 5mg are - Dizziness, Headache, Fatigue, Edema (fluid retention), Flushing, Palpitations (irregular heartbeat), Rash, Nausea, Constipation, and Diarrhea.? Q: What is the dosage of Amdocal 5mg? You should take Amdocal 5mg on your doctor?s advice. Usually, the starting dose is 5mg once a day. Your doctor can increase the dose depending on your response to the medication.Sku: 1736108110-4651
Amdocal5 mg
₦4,537.50Original price was: ₦4,537.50.₦4,083.75Current price is: ₦4,083.75.₦4,537.50Original price was: ₦4,537.50.₦4,083.75Current price is: ₦4,083.75. Add to basket Quick View -
SaleAmeloss 5 mgDonepezil is indicated for the symptomatic treatment of mild to moderate dementia of Alzheimer's type.Theropeutic ClassDrugs for DementiaPharmacologyAmeloss 5 mg is a centrally acting anticholinesterase agent. It binds reversibly with acetylcholinesterase and inactivates it, thus inhibiting hydrolysis of acetylcholine. As a result the concentration of acetylcholine increases at cholinergic synapses in the brain.Dosage & Administration of Ameloss 5 mg5 mg once daily orally at bed time. The 5 mg/day dose should be maintained for at least one month in order to allow the earliest clinical responses to treatment to be assessed and to allow steady-state concentrations of Ameloss 5 mg to be achieved. Following a one-month clinical assessment of treatment at 5 mg/day, the dose can be increased to 10 mg/day (once-a-day dosing). Since food does not affect the rate or extent of absorption of donepezil, it can be administered with or without food.Dosage of Ameloss 5 mg5 mg once daily orally at bed time. The 5 mg/day dose should be maintained for at least one month in order to allow the earliest clinical responses to treatment to be assessed and to allow steady-state concentrations of Ameloss 5 mg to be achieved. Following a one-month clinical assessment of treatment at 5 mg/day, the dose can be increased to 10 mg/day (once-a-day dosing). Since food does not affect the rate or extent of absorption of donepezil, it can be administered with or without food.Interaction of Ameloss 5 mgDrugs with anticholinergic properties and which cross into the brain, such as atropine, benztropine produce the opposite effects of Donepezil and should be avoided during therapy with donepezil. Medication with carbamazepine, dexamethasone, phenobarbital, phenytoin may reduce the effect of donepezil whereas ketoconazole, quinidine, cimetidine may increase the effects.ContraindicationsDonepezil is contraindicated in patients with known hypersensitivity to Ameloss 5 mg or to piperidine derivatives.Side Effects of Ameloss 5 mgGenerally well tolerated but some patients may experience nausea, vomiting & diarrhoea. These adverse events are of mild intensity and transient, resolving during continued treatment without the need for dose modification. Less frequent side effects are insomnia, fatigue, anorexia, muscle cramps, generalized seizure etc.Pregnancy & LactationThere are no adequate and well controlled studies in pregnant woman. Donepezil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Ameloss 5 mg is secreted in human breast milk or not. Donepezil is not indicated in nursing mother.Precautions & WarningsCaution should be taken in sick sinus syndrome or other supraventricular conduction abnormalities, patients at risk of developing peptic ulcers, asthma, obstructive airway disease and during anaesthetic procedure.Overdose Effects of Ameloss 5 mgSymptoms of overdose includes nausea, vomiting, salivation. Over dosage with very high dose of donepezil may result in cholinergic crisis characterized by severe nausea, vomiting, salivation, bradycardia, hypotension, increasing muscle weakness, respiratory depression, collapse and convulsion. As in any case of over dose, general supportive measures should be utilized. In case of cholinergic crisis hospitalization of the patient is required.Storage ConditionsKeep below 30?C temperature, away from light & moisture. Keep out of the reach of children.Use In Special PopulationsRenal & hepatic impairment: A similar dose schedule can be followed for patients with renal or mild to moderate hepatic impairment as clearance of Ameloss 5 mg is not affected by these conditions.Use in children: There are no adequate and well controlled trials in document to safety and efficacy of Donepezil hydrochloride in any illness occurring in children. Donepezil is not recommended for use in children.Drug ClassesDrugs for DementiaMode Of ActionAmeloss 5 mg is a centrally acting anticholinesterase agent. It binds reversibly with acetylcholinesterase and inactivates it, thus inhibiting hydrolysis of acetylcholine. As a result the concentration of acetylcholine increases at cholinergic synapses in the brain.PregnancyThere are no adequate and well controlled studies in pregnant woman. Donepezil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Ameloss 5 mg is secreted in human breast milk or not. Donepezil is not indicated in nursing mother.Pediatric UsesRenal & hepatic impairment: A similar dose schedule can be followed for patients with renal or mild to moderate hepatic impairment as clearance of Ameloss 5 mg is not affected by these conditions.Use in children: There are no adequate and well controlled trials in document to safety and efficacy of Donepezil hydrochloride in any illness occurring in children. Donepezil is not recommended for use in children.Sku: 1736104866-3683
Ameloss5 mg
₦5,500.00Original price was: ₦5,500.00.₦4,950.00Current price is: ₦4,950.00.₦5,500.00Original price was: ₦5,500.00.₦4,950.00Current price is: ₦4,950.00. Add to basket Quick View -
SaleAmilin Plus 12.5 mg+5 mgThis is indicated for the treatment of patients with moderate to severe depression associated with moderate to severe anxiety. Symptoms likely to respond in the first week of treatment include insomnia, feelings of guilt or worthlessness, agitation, psychic and somatic anxiety, suicidal ideation and anorexia.Dosage of Amilin Plus 12.5 mg+5 mgOptimum dosage varies with the severity of the symptoms and the response of the individual patient. When a satisfactory response is obtained, the dosage should be reduced to the smallest amount needed to maintain the remission. The larger portion of the total daily dose may be taken at bedtime. In some patients, a single dose at bedtime may be sufficient. This tablet in an initial dosage of 3 or 4 tablets daily in divided doses is satisfactory. Or directed by the physican.Interaction of Amilin Plus 12.5 mg+5 mgBecause of its Amitriptyline component, this preparation may block the antihypertensive action of guanethidine or compounds with a similar mechanism of action.ContraindicationsThis is contraindicated in patients with hypersensitivity to either benzodiazepines or tricyclic antidepressants. It should not be given concomitantly with a monoamine oxidase inhibitor.Side Effects of Amilin Plus 12.5 mg+5 mgSide effects: Many symptoms common to the depressive state, such as anorexia, fatigue, weakness, restlessness and lethargy, have been reported as side effects of treatment with this preparation.Adverse reactions: Most frequently reported were drowsiness, dry mouth, constipation, blurred vision, dizziness and bloating. Less commonly included vivid dreams, impotence, tremor, confusion and nasal congestion.Precautions & WarningsUse with caution in patients with a history of seizures. Close supervision is required when this preparation is given to hyperthyroid patients or those on thyroid medication. The usual precautions should be observed when treating patients with impaired renal or hepatic function. All pediatric patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality and unusual changes in behavior.Overdose Effects of Amilin Plus 12.5 mg+5 mgDeaths may occur from overdosage with this class of drugs. Critical manifestations of Amitriptyline overdose include cardiac dysrhythmias, severe hypotension, convulsions and CNS depression, including coma. Manifestations of benzodiazepine overdosage include somnolence, confusion, coma and diminished reflexes.Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of Chlordiazepoxide. Generally, milder withdrawal symptoms (eg, dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Withdrawal symptoms (e.g., nausea, headache and malaise) have also been reported in association with abrupt Amitriptyline discontinuation.Storage ConditionsStore in a cool and dry place, protected from light. Keep all medicines out of reach of the children.Drug ClassesCombined anxiolytics & anti-depressant drugsMode Of ActionAmitriptyline is a tricyclic antidepressant and Chlordiazepoxide is an anxiolytic. Amitriptyline inhibits the reuptake of norepinephrine and serotonin in the brain. This interference with the reuptake is responsible for the antidepressant activity of Amitriptyline. Chlordiazepoxide works by enhancing GABA-mediated chloride influx through GABA receptor channels, causing membrane hyperpolarization. The net neuro-inhibitory effects result in the observed sedative and anxiolytic effect.PregnancySafe use of this preparation during pregnancy and lactation has not been established.Pediatric UsesPediatric Use: Safety and effectiveness in the pediatric population have not been established. Anyone considering the use of Chlordiazepoxide and Amitriptyline Hydrochloride Tablets in a child or adolescent must balance the potential risks with the clinical need.Geriatric Use: In elderly and debilitated patients it is recommended that dosage be limited to the smallest effective amount to preclude the development of ataxia, over sedation, confusion or anticholinergic effects.Sku: 1736097850-1623
Amilin Plus12.5 mg+5 mg
₦5,390.00Original price was: ₦5,390.00.₦4,851.00Current price is: ₦4,851.00.₦5,390.00Original price was: ₦5,390.00.₦4,851.00Current price is: ₦4,851.00. Add to basket Quick View -
SaleAmilin 10 mgAmilin 10 mg is indicated in- Depressive Illness: particularly where sedation is required. Nocturnal Enuresis in children. Prophylaxis of Migraine. Tension Headache. Chronic Pain.Theropeutic ClassTricyclic Anti-depressantPharmacologyAmitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system.Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of Amitriptyline.Dosage & Administration of Amilin 10 mgDepression : Adults: Initially 50-70 mg a day in divided dose or as a single dose at night at bed time. Elderly and adolescents: 25-50 mg daily in divided doses or as single dose at bed time. Dose can be increased gradually as necessary to a maximum of 150-200 mg. Usual maintenance dose is 50-100 mg daily. Nocturnal enuresis: 6-10 years: 10-20 mg at bed time. 11-16 years: 25-50 mg at bed time for up to 3 months and gradually withdrawn.Dosage of Amilin 10 mgDepression: Initially 75 mg (Elderly and Adolescents 30-75 mg) daily in divided doses or as a single dose at bedtime increased gradually as necessary to 150-200 mg; Child under 16 years not recommended for depression.Nocturnal Enuresis: Child 7-10 years: 10-20 mg, 11-16 years: 25-50 mg at night; max. period of treatment (including gradual withdrawal) 3 months-full physical examination before the further course. Prophylaxis of Migraine: 100 mg daily.Tension Headache: 10-25 mg three times daily.Interaction of Amilin 10 mgTCA enhances the sedative effect of alcohol and opioid analgesics. When TCA is used with Moxifloxacin or Terfenadine, it increases the risk of ventricular arrhythmias. Disulfirum and Cimetidine inhibit the metabolism of Amitriptyline. When TCA is used with diuretics, it enhances the risk of postural hypotension.ContraindicationsAmitriptyline is contraindicated in myocardial infarction, arrythmias, particularly heart block of any degree, mania and severe liver disease. Initially, sedation may affect the ability to drive or operate machinery.Side Effects of Amilin 10 mgAnticholinergic: Excessive perspiration, dry mouth, blurred vision, hyperpyrexia, urinary retention and urinary tract dilatation. Cardiovascular reactions: Hypotension, syncope, postural hypotension, hypertension, tachycardia, palpitations, myocardial infarction, etc. CNS and Neuromuscular: Confusional states, disturbed concentration, disorientation, delusions, etc. Allergic: Skin rash, urticaria, photosensitization, etc. Haematological: Bone-marrow depression. Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, unpleasant taste, weight loss, diarrhoea, constipation, etc. Endocrine: Testicular swelling, gynaecomastia, breast enlargement, galactorrhoea, etc.Pregnancy & LactationPregnancy Category C. Amilin 10 mg is not recommended during pregnancy, especially during the first and third trimester because the safety of Amitriptyline has not been established yet. Amitriptyline is detectable in breast milk. Because of the serious adverse reactions in infants from Amitriptyline, a decision should be made whether to continue breast feeding or discontinue the drugPrecautions & WarningsIt should be used with caution in patients with a history of epilepsy, glaucoma, urinary retention, cardiac disease, diabetes, pregnancy, hepatic impairment, thyroid disease, increased intra-ocular pressure and psychoses (may aggravate mania).Storage ConditionsStore in a cool and dry place, below 30?C. Protect from light and moisture.Drug ClassesTricyclic Anti-depressantMode Of ActionThe mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown.PregnancyPregnancy Category C. Amitriptyline has been shown to cross the placenta. Amitriptyline should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Amitriptyline is excreted into breast milk. Because of the potential for serious adverse reactions in nursing infants from Amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Sku: 1736107663-4516
Amilin10 mg
₦654.50Original price was: ₦654.50.₦589.05Current price is: ₦589.05. -
SaleAmilin 25 mgAmilin 25 mg is indicated in- Depressive Illness: particularly where sedation is required. Nocturnal Enuresis in children. Prophylaxis of Migraine. Tension Headache. Chronic Pain.Theropeutic ClassTricyclic Anti-depressantPharmacologyAmitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system.Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of Amitriptyline.Dosage & Administration of Amilin 25 mgDepression : Adults: Initially 50-70 mg a day in divided dose or as a single dose at night at bed time. Elderly and adolescents: 25-50 mg daily in divided doses or as single dose at bed time. Dose can be increased gradually as necessary to a maximum of 150-200 mg. Usual maintenance dose is 50-100 mg daily. Nocturnal enuresis: 6-10 years: 10-20 mg at bed time. 11-16 years: 25-50 mg at bed time for up to 3 months and gradually withdrawn.Dosage of Amilin 25 mgDepression: Initially 75 mg (Elderly and Adolescents 30-75 mg) daily in divided doses or as a single dose at bedtime increased gradually as necessary to 150-200 mg; Child under 16 years not recommended for depression.Nocturnal Enuresis: Child 7-10 years: 10-20 mg, 11-16 years: 25-50 mg at night; max. period of treatment (including gradual withdrawal) 3 months-full physical examination before the further course. Prophylaxis of Migraine: 100 mg daily.Tension Headache: 10-25 mg three times daily.Interaction of Amilin 25 mgTCA enhances the sedative effect of alcohol and opioid analgesics. When TCA is used with Moxifloxacin or Terfenadine, it increases the risk of ventricular arrhythmias. Disulfirum and Cimetidine inhibit the metabolism of Amitriptyline. When TCA is used with diuretics, it enhances the risk of postural hypotension.ContraindicationsAmitriptyline is contraindicated in myocardial infarction, arrythmias, particularly heart block of any degree, mania and severe liver disease. Initially, sedation may affect the ability to drive or operate machinery.Side Effects of Amilin 25 mgAnticholinergic: Excessive perspiration, dry mouth, blurred vision, hyperpyrexia, urinary retention and urinary tract dilatation. Cardiovascular reactions: Hypotension, syncope, postural hypotension, hypertension, tachycardia, palpitations, myocardial infarction, etc. CNS and Neuromuscular: Confusional states, disturbed concentration, disorientation, delusions, etc. Allergic: Skin rash, urticaria, photosensitization, etc. Haematological: Bone-marrow depression. Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, unpleasant taste, weight loss, diarrhoea, constipation, etc. Endocrine: Testicular swelling, gynaecomastia, breast enlargement, galactorrhoea, etc.Pregnancy & LactationPregnancy Category C. Amilin 25 mg is not recommended during pregnancy, especially during the first and third trimester because the safety of Amitriptyline has not been established yet. Amitriptyline is detectable in breast milk. Because of the serious adverse reactions in infants from Amitriptyline, a decision should be made whether to continue breast feeding or discontinue the drugPrecautions & WarningsIt should be used with caution in patients with a history of epilepsy, glaucoma, urinary retention, cardiac disease, diabetes, pregnancy, hepatic impairment, thyroid disease, increased intra-ocular pressure and psychoses (may aggravate mania).Storage ConditionsStore in a cool and dry place, below 30?C. Protect from light and moisture.Drug ClassesTricyclic Anti-depressantMode Of ActionThe mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown.PregnancyPregnancy Category C. Amitriptyline has been shown to cross the placenta. Amitriptyline should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Amitriptyline is excreted into breast milk. Because of the potential for serious adverse reactions in nursing infants from Amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Sku: 1736107517-4472
Amilin25 mg
₦1,347.50Original price was: ₦1,347.50.₦1,212.75Current price is: ₦1,212.75.₦1,347.50Original price was: ₦1,347.50.₦1,212.75Current price is: ₦1,212.75. Add to basket Quick View -
SaleAmipride 50 mgAmipride 50 mg tablet is indicated for the treatment of acute and chronic schizophrenic disorders, in which positive symptoms (such as delusions, hallucinations, thought disorders) and/or negative symptoms (such as blunted affect, emotional and social withdrawal) are prominent, including ... Read moreAmipride 50 mg tablet is indicated for the treatment of acute and chronic schizophrenic disorders, in which positive symptoms (such as delusions, hallucinations, thought disorders) and/or negative symptoms (such as blunted affect, emotional and social withdrawal) are prominent, including patients characterized by predominant negative symptoms.Amipride 50 mg injection is indicated in adults for: Prevention of postoperative nausea and vomiting (PONV), either alone or in combination with an antiemetic of a different class. Treatment of postoperative nausea and vomiting (PONV) in patients who have received antiemetic prophylaxis with an agent of a different class or have not received prophylaxis. Theropeutic ClassAtypical neuroleptic drugsPharmacologyAmipride 50 mg binds selectively to the human dopaminergic D2 and D3 receptor subtypes without any affinity for D1, D4 and D5 receptor subtypes. Unlike classical and atypical neuroleptics, Amipride 50 mg displays low affinity for serotonin, ?- adrenergic, histamine receptor subtypes, muscarinic receptors and sigma sites. In the rodent, it preferentially blocks post synaptic D2 receptors located in the limbic structures as compared to those in the striatum as indicated by its reversal of d-amphetamineinduced hyperactivity without affecting stereotypies. In addition, it does not induce catalepsy and it does not produce D2 hypersensitivity after repeated treatment.Moreover, it preferentially blocks pre-synaptic D2/D3 dopamine receptors, producing dopamine release responsible for its disinhibitory effects. This atypical pharmacological profile may explain Amipride 50 mg?s antipsychotic effect at higher doses through post-synaptic dopamine receptor blockade located in the limbic areas and its efficacy against negative symptoms, at lower doses, through presynaptic dopamine receptor blockade. In addition, the reduced tendency of Amipride 50 mg to produce extrapyramidal side effects may be related to its preferential limbic activity.Dosage & Administration of Amipride 50 mgFor acute psychotic episodes, oral doses between 400 mg/d and 800 mg/d are recommended. In individual cases, the daily dose may be increased up to 1200 mg/d. Doses above 1200 mg/d have not been extensively evaluated for safety and therefore should not be used. Doses above 800 mg/d have not been shown to be superior to lower doses and may increase the incidence of adverse events. No specific titration is required when initiating the treatment with Amipride 50 mg. Doses should be adjusted according to individual response. Doses should preferably be administered before meals. Amipride 50 mg should be administered twice daily for doses above 400 mg. For patients with mixed positive and negative symptoms, doses should be adjusted to obtain optimal control of positive symptoms. Maintenance treatment should be established individually with the minimally effective dose. For patients characterized by predominant negative symptoms, oral doses between 50 mg/d and 300 mg/d are recommended. Doses should be adjusted individually.Dosage of Amipride 50 mgOral dose: For acute psychotic episodes, oral doses between 400 mg/d and 800 mg/d are recommended. In individual cases, the daily dose may be increased up to 1200 mg/d. Doses above 1200 mg/d have not been extensively evaluated for safety and therefore should not be used. Doses above 800 mg/d have not been shown to be superior to lower doses and may increase the incidence of adverse events. No specific titration is required when initiating the treatment with Amipride 50 mg. Doses should be adjusted according to individual responses. Doses should preferably be administered before meals. Amipride 50 mg should be administered twice daily for doses above 400 mg. For patients with mixed positive and negative symptoms, doses should be adjusted to obtain optimal control of positive symptoms. Maintenance treatment should be established individually with the minimally effective dose. For patients characterized by predominant negative symptoms, oral doses between 50 mg/d and 300 mg/d are recommended. Doses should be adjusted individually.Injectable dose: The recommended injectable adult dosage of Amipride 50 mg and infusion rate by indication is shown in the table below: Prevention of postoperative nausea and vomiting: 5 mg as a single intravenous injection infused over 1 to 2 minutes at the time of induction of anesthesia. Treatment of postoperative nausea and vomiting: 10 mg as a single intravenous injection infused over 1 to 2 minutes in the event of nausea and/or vomiting after a surgical procedure. ContraindicationsHypersensitivity to the active ingredient or to other ingredients of the product. Concomitant prolactin-dependent tumours e.g. pituitary gland prolactinomas and breast cancer. Phaeochromocytoma. Children up to puberty. Lactation.Pregnancy & Lactationpregnancy Category C. The safety of Amipride 50 mg during human pregnancy has not been established, and therefore use of Amipride 50 mg is not recommended during pregnancy and in women of child bearing potential not using effective contraception, unless the benefits justify the potential risks and the administered dose and duration of treatment should be as low and as short as possible. Amipride 50 mg has been found in the breast milk of treated women. Breast-feeding is contraindicated.Precautions & WarningsNeuroleptic Malignant Syndrome (NMS) is a potentially fatal syndrome that has been reported in association with anti psychotic medicines, including Amipride 50 mg. Neuroleptic malignant syndrome is characterised by hyperthermia, muscle rigidity, autonomic instability, and elevated CPK, may occur. In the event of any symptoms which could suggest NMS, in particular hyperthermia, particularly with high daily doses, all antipsychotic medicines including Amipride 50 mg should be discontinued. Amipride 50 mg can lower the seizure threshold. Therefore patients with a history of seizures should be closely monitored during Amipride 50 mg therapy.Storage ConditionsKeep below 30?C temperature, away from light & moisture. Keep out of the reach of children.Use In Special PopulationsElderly: Amipride 50 mg should be used with particular caution because of a possible risk of hypotension or sedation.Children: The efficacy and safety of Amipride 50 mg from puberty to the age of 18 years have not been established: there are limited data available on the use of Amipride 50 mg in adolescents in schizophrenia. Therefore, the use of Amipride 50 mg from puberty to the age of 18 years is not recommended. In children up to puberty, the use of Amipride 50 mg is contraindicatedUse in hepatic impairment: The impact of hepatic impairment on hepatic metabolism and hepato-biliary excretion of Amipride 50 mg has not been studied. Amipride 50 mg should be used with caution in patients with moderate or severe hepatic impairment.Use in renal impairment: Amipride 50 mg is eliminated by the renal route. In cases of renal insufficiency, the dose should be decreased and intermittent treatment should be consideredDrug ClassesAtypical neuroleptic drugsMode Of ActionAmipride 50 mg binds selectively to the human dopaminergic D2 and D3 receptor subtypes without any affinity for D1, D4 and D5 receptor subtypes. Unlike classical and atypical neuroleptics, Amipride 50 mg displays low affinity for serotonin, ?- adrenergic, histamine receptor subtypes, muscarinic receptors and sigma sites. In the rodent, it preferentially blocks post synaptic D2 receptors located in the limbic structures as compared to those in the striatum as indicated by its reversal of d-amphetamineinduced hyperactivity without affecting stereotypies. In addition, it does not induce catalepsy and it does not produce D2 hypersensitivity after repeated treatment.Moreover, it preferentially blocks pre-synaptic D2/D3 dopamine receptors, producing dopamine release responsible for its disinhibitory effects. This atypical pharmacological profile may explain Amipride 50 mg?s antipsychotic effect at higher doses through post-synaptic dopamine receptor blockade located in the limbic areas and its efficacy against negative symptoms, at lower doses, through presynaptic dopamine receptor blockade. In addition, the reduced tendency of Amipride 50 mg to produce extrapyramidal side effects may be related to its preferential limbic activity.Pregnancypregnancy Category C. The safety of Amipride 50 mg during human pregnancy has not been established, and therefore use of Amipride 50 mg is not recommended during pregnancy and in women of child bearing potential not using effective contraception, unless the benefits justify the potential risks and the administered dose and duration of treatment should be as low and as short as possible. Amipride 50 mg has been found in the breast milk of treated women. Breast-feeding is contraindicated.Pediatric UsesElderly: Amipride 50 mg should be used with particular caution because of a possible risk of hypotension or sedation.Children: The efficacy and safety of Amipride 50 mg from puberty to the age of 18 years have not been established: there are limited data available on the use of Amipride 50 mg in adolescents in schizophrenia. Therefore, the use of Amipride 50 mg from puberty to the age of 18 years is not recommended. In children up to puberty, the use of Amipride 50 mg is contraindicatedUse in hepatic impairment: The impact of hepatic impairment on hepatic metabolism and hepato-biliary excretion of Amipride 50 mg has not been studied. Amipride 50 mg should be used with caution in patients with moderate or severe hepatic impairment.Use in renal impairment: Amipride 50 mg is eliminated by the renal route. In cases of renal insufficiency, the dose should be decreased and intermittent treatment should be consideredSku: 1736106296-4104
Amipride50 mg
₦11,000.00Original price was: ₦11,000.00.₦10,120.00Current price is: ₦10,120.00.₦11,000.00Original price was: ₦11,000.00.₦10,120.00Current price is: ₦10,120.00. Add to basket Quick View -
SaleAmit 10 mgAmit 10 mg is indicated in- Depressive Illness: particularly where sedation is required. Nocturnal Enuresis in children. Prophylaxis of Migraine. Tension Headache. Chronic Pain.Theropeutic ClassTricyclic Anti-depressantPharmacologyAmitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system.Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of Amitriptyline.Dosage & Administration of Amit 10 mgDepression : Adults: Initially 50-70 mg a day in divided dose or as a single dose at night at bed time. Elderly and adolescents: 25-50 mg daily in divided doses or as single dose at bed time. Dose can be increased gradually as necessary to a maximum of 150-200 mg. Usual maintenance dose is 50-100 mg daily. Nocturnal enuresis: 6-10 years: 10-20 mg at bed time. 11-16 years: 25-50 mg at bed time for up to 3 months and gradually withdrawn.Dosage of Amit 10 mgDepression: Initially 75 mg (Elderly and Adolescents 30-75 mg) daily in divided doses or as a single dose at bedtime increased gradually as necessary to 150-200 mg; Child under 16 years not recommended for depression.Nocturnal Enuresis: Child 7-10 years: 10-20 mg, 11-16 years: 25-50 mg at night; max. period of treatment (including gradual withdrawal) 3 months-full physical examination before the further course. Prophylaxis of Migraine: 100 mg daily.Tension Headache: 10-25 mg three times daily.Interaction of Amit 10 mgTCA enhances the sedative effect of alcohol and opioid analgesics. When TCA is used with Moxifloxacin or Terfenadine, it increases the risk of ventricular arrhythmias. Disulfirum and Cimetidine inhibit the metabolism of Amitriptyline. When TCA is used with diuretics, it enhances the risk of postural hypotension.ContraindicationsAmitriptyline is contraindicated in myocardial infarction, arrythmias, particularly heart block of any degree, mania and severe liver disease. Initially, sedation may affect the ability to drive or operate machinery.Side Effects of Amit 10 mgAnticholinergic: Excessive perspiration, dry mouth, blurred vision, hyperpyrexia, urinary retention and urinary tract dilatation. Cardiovascular reactions: Hypotension, syncope, postural hypotension, hypertension, tachycardia, palpitations, myocardial infarction, etc. CNS and Neuromuscular: Confusional states, disturbed concentration, disorientation, delusions, etc. Allergic: Skin rash, urticaria, photosensitization, etc. Haematological: Bone-marrow depression. Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, unpleasant taste, weight loss, diarrhoea, constipation, etc. Endocrine: Testicular swelling, gynaecomastia, breast enlargement, galactorrhoea, etc.Pregnancy & LactationPregnancy Category C. Amit 10 mg is not recommended during pregnancy, especially during the first and third trimester because the safety of Amitriptyline has not been established yet. Amitriptyline is detectable in breast milk. Because of the serious adverse reactions in infants from Amitriptyline, a decision should be made whether to continue breast feeding or discontinue the drugPrecautions & WarningsIt should be used with caution in patients with a history of epilepsy, glaucoma, urinary retention, cardiac disease, diabetes, pregnancy, hepatic impairment, thyroid disease, increased intra-ocular pressure and psychoses (may aggravate mania).Storage ConditionsStore in a cool and dry place, below 30?C. Protect from light and moisture.Drug ClassesTricyclic Anti-depressantMode Of ActionThe mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown.PregnancyPregnancy Category C. Amitriptyline has been shown to cross the placenta. Amitriptyline should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Amitriptyline is excreted into breast milk. Because of the potential for serious adverse reactions in nursing infants from Amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Sku: 1736104444-3557
Amit10 mg
₦467.50Original price was: ₦467.50.₦420.75Current price is: ₦420.75. -
SaleAmit 25 mgAmit 25 mg is indicated in- Depressive Illness: particularly where sedation is required. Nocturnal Enuresis in children. Prophylaxis of Migraine. Tension Headache. Chronic Pain.Theropeutic ClassTricyclic Anti-depressantPharmacologyAmitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system.Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically, this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of Amitriptyline.Dosage & Administration of Amit 25 mgDepression : Adults: Initially 50-70 mg a day in divided dose or as a single dose at night at bed time. Elderly and adolescents: 25-50 mg daily in divided doses or as single dose at bed time. Dose can be increased gradually as necessary to a maximum of 150-200 mg. Usual maintenance dose is 50-100 mg daily. Nocturnal enuresis: 6-10 years: 10-20 mg at bed time. 11-16 years: 25-50 mg at bed time for up to 3 months and gradually withdrawn.Dosage of Amit 25 mgDepression: Initially 75 mg (Elderly and Adolescents 30-75 mg) daily in divided doses or as a single dose at bedtime increased gradually as necessary to 150-200 mg; Child under 16 years not recommended for depression.Nocturnal Enuresis: Child 7-10 years: 10-20 mg, 11-16 years: 25-50 mg at night; max. period of treatment (including gradual withdrawal) 3 months-full physical examination before the further course. Prophylaxis of Migraine: 100 mg daily.Tension Headache: 10-25 mg three times daily.Interaction of Amit 25 mgTCA enhances the sedative effect of alcohol and opioid analgesics. When TCA is used with Moxifloxacin or Terfenadine, it increases the risk of ventricular arrhythmias. Disulfirum and Cimetidine inhibit the metabolism of Amitriptyline. When TCA is used with diuretics, it enhances the risk of postural hypotension.ContraindicationsAmitriptyline is contraindicated in myocardial infarction, arrythmias, particularly heart block of any degree, mania and severe liver disease. Initially, sedation may affect the ability to drive or operate machinery.Side Effects of Amit 25 mgAnticholinergic: Excessive perspiration, dry mouth, blurred vision, hyperpyrexia, urinary retention and urinary tract dilatation. Cardiovascular reactions: Hypotension, syncope, postural hypotension, hypertension, tachycardia, palpitations, myocardial infarction, etc. CNS and Neuromuscular: Confusional states, disturbed concentration, disorientation, delusions, etc. Allergic: Skin rash, urticaria, photosensitization, etc. Haematological: Bone-marrow depression. Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, unpleasant taste, weight loss, diarrhoea, constipation, etc. Endocrine: Testicular swelling, gynaecomastia, breast enlargement, galactorrhoea, etc.Pregnancy & LactationPregnancy Category C. Amit 25 mg is not recommended during pregnancy, especially during the first and third trimester because the safety of Amitriptyline has not been established yet. Amitriptyline is detectable in breast milk. Because of the serious adverse reactions in infants from Amitriptyline, a decision should be made whether to continue breast feeding or discontinue the drugPrecautions & WarningsIt should be used with caution in patients with a history of epilepsy, glaucoma, urinary retention, cardiac disease, diabetes, pregnancy, hepatic impairment, thyroid disease, increased intra-ocular pressure and psychoses (may aggravate mania).Storage ConditionsStore in a cool and dry place, below 30?C. Protect from light and moisture.Drug ClassesTricyclic Anti-depressantMode Of ActionThe mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown.PregnancyPregnancy Category C. Amitriptyline has been shown to cross the placenta. Amitriptyline should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Amitriptyline is excreted into breast milk. Because of the potential for serious adverse reactions in nursing infants from Amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Sku: 1736103795-3368
Amit25 mg
₦96.80Original price was: ₦96.80.₦86.90Current price is: ₦86.90. -
SaleSku: 1736105649-3913
Amlevo2.5 mg
₦3,850.00Original price was: ₦3,850.00.₦3,503.50Current price is: ₦3,503.50.₦3,850.00Original price was: ₦3,850.00.₦3,503.50Current price is: ₦3,503.50. Add to basket Quick View -
SaleSku: 1736104052-3443
Amlevo5 mg
₦5,500.00Original price was: ₦5,500.00.₦5,005.00Current price is: ₦5,005.00.₦5,500.00Original price was: ₦5,500.00.₦5,005.00Current price is: ₦5,005.00. Add to basket Quick View -
SaleAmlocard Plus 5 mg+50 mgAmlodipine & Atenolol is indicated in: Hypertension not controlled by monotherapy Angina pectoris & hypertension co-existing diseases Post MI patients Refractory angina pectoris where nitrate therapy has failed Theropeutic ClassCombined antihypertensive preparationsPharmacologyAmlodipine is a dihydropyridine calcium antagonist that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle; it has a greater effect on vascular muscle than on cardiac muscle. Amlodipine is a peripheral vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. Amlodipine reduces tone, decreases coronary vasoreactivity and lowers cardiac demand by reducing afterload. Atenolol is a cardio selective beta blocker. The cardio selectivity is dose related. Atenolol causes a reduction in blood pressure by lowering cardiac output, decreasing the plasma renin activity and sympathetic outflow from CNS. Atenolol also causes a reduction in myocardial oxygen demand by virtue of its negative inotropic and negative chronotropic effects.Dosage & Administration of Amlocard Plus 5 mg+50 mgThe recommended dosage is one tablet daily of (Amlodipine 5 mg & Atenolol 50 mg) or (Amlodipine 5 mg & Atenolol 25 mg). Depending upon the therapeutic response, titration of the dosage is recommended. In elderly patients, it is advisable to initiate the therapy with ? tablet of fixed dose combination of Amlodipine & Atenolol (i.e. 2.5 mg of Amlodipine & 25 mg Atenolol).Interaction of Amlocard Plus 5 mg+50 mgAmlodipine has been safely administered with thiazide diuretics, beta blockers, alpha blockers, angiotensin converting enzyme inhibitors, long-acting nitrates, sublingual glyceryl trinitrate, non-steroidal anti-inflammatory agents, antibiotics, and oral hypoglycemic agents. In vitro data from studies with human plasma indicate that amlodipine has no effect on protein binding of the drugs tested (Digoxin, Phenytoin, Warfarin, or Indomethacin). Atenolol reduces the clearance of Disopyramide by 20%. Additive negative inotropic effects on the heart may be produced. At doses of 1 gm and above, Ampicillin may reduce Atenolol levels. Beta-blockers may decrease tissue sensitivity to Insulin and inhibit Insulin secretion, e.g. in response to oral antidiabetics. Atenolol has less potential for these actions.ContraindicationsHypersensitivity to either component, sinus bradycardia, second and higher degrees of heart block, cardiogenic shock, hypotension, congestive heart failure, poor left ventricular function.Side Effects of Amlocard Plus 5 mg+50 mgThe combination of Amlodipine and Atenolol is well tolerated. Overall side effects include fatigue, headache, edema, nausea, drowsiness, anxiety and depression.Pregnancy & LactationAtenolol crosses the placenta. So it is contraindicated in pregnancy. It should be avoided during lactation.Precautions & WarningsAtenolol may mask the symptoms of hyperthyroidism. It may also mask the symptoms of hypoglycaemia, as well as enhance the effects of hypoglycaemic agents in patients with diabetes mellitus.Storage ConditionsStore in a cool dry place protected from light. Keep out of reach of children.Sku: 1736103440-3267
Amlocard Plus5 mg+50 mg
₦5,390.00Original price was: ₦5,390.00.₦4,851.00Current price is: ₦4,851.00.₦5,390.00Original price was: ₦5,390.00.₦4,851.00Current price is: ₦4,851.00. Add to basket Quick View -
SaleAmlocard 5 mgAmlocard 5 mg is a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of Hypertension and Coronary Artery Disease (such as Chronic Stable Angina, Vasospastic Angina and Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction 1.0% are headache, fatigue, nausea, abdominal pain and somnolence.Pregnancy & LactationPregnancy Category C. There are no adequate and well-controlled studies of Amlocard 5 mg in pregnant women. Amlocard 5 mg should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Amlocard 5 mg is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while Amlocard 5 mg is administered.Precautions & WarningsSymptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of Amlocard 5 mg, particularly in patients with severe obstructive coronary artery disease. Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment.Overdose Effects of Amlocard 5 mgIn humans, experience with intentional overdose is limited.Symptoms: Available data suggest that large overdosage could result in excessive peripheral vasodilatation and possibly reflex tachycardia. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.Management: Clinically significant hypotension due to Amlocard 5 mg overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output.?A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade. Gastric lavage may be worthwhile in some cases. In healthy volunteers the use of charcoal up to 2 hours after administration of Amlocard 5 mg 10 mg has been shown to reduce the absorption rate of Amlocard 5 mg. Since Amlocard 5 mg is highly protein-bound, dialysis is not likely to be of benefit.Storage Conditionskeep in a dry place away from light and heat. Keep out of the reach of children.Use In Special PopulationsChildren with hypertension from 6 years to 17 years of age: 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients.Children under 6 years old: ?The effect of Amlocard 5 mg on blood pressure in patients less than 6 years of age is not known.Elderly: Amlocard 5 mg used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care.Renal impairment: Changes in Amlocard 5 mg plasma concentrations are not correlated with degree of renal impairment, therefore the normal dosage is recommended. Amlocard 5 mg is not dialysable.Hepatic impairment: Dosage recommendations have not been established in patients with mild to moderate hepatic impairment; therefore dose selection should be cautions and should start at the lower end of the dosing range. The pharmacokinetics of Amlocard 5 mg have not been studied in severe hepatic impairment. Amlocard 5 mg should be initiated at the lowest dose (2.5 mg once daily) and titrated slowly in patients with severe hepatic impairment.Sku: 1736103080-3164
Amlocard5 mg
₦3,850.00Original price was: ₦3,850.00.₦3,465.00Current price is: ₦3,465.00.₦3,850.00Original price was: ₦3,850.00.₦3,465.00Current price is: ₦3,465.00. Add to basket Quick View