-
TRAKLOT 500 Tablet 6?s ₦30,600.00 QTY: 1
-
Quikhaler Dpi Device 1?s ₦40,800.00 QTY: 1
-
GLUCONORM VG 1MG TAB 15`S ₦5,238.50 QTY: 1
-
CEFF FORTE PREMIX SYP ₦3,807.00 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
60241–60256 of 173246 Results
-
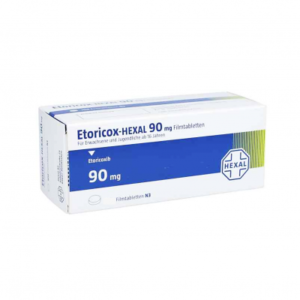
ETORICOX - HEXAL 90 MG ( ETORICOXIB ) 10 FILM-COATED TABLETSSku: 1720434726-609
ETORICOX – HEXAL 90 MG ( ETORICOXIB ) 10 FILM-COATED TABLETS
₦520.00 -
-
The name of the medicine is Etoricoxib 120mg tabs 10s film-coated tablets. The active substance etoricoxib which belongs to a group of medicines called selective COX-2 inhibitors. These belong to a family of medicines called non-steroidal anti-inflammatory drugs (NSAIDs).Sku: 1715468248-554
ETORICOXIB 120MG (ZETCOXI) TABS 10S
₦100.00 -
-
Etorocoxib tabs is indicated for the treatment of rheumatoid arthritis, psoriatic arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and goutSku: 1713648353-380
Etoricoxib 60mg Atoxia Tablets 10?s
₦100.00 -
-
etoricoxib is a non steroidal anti inflammatory drug,used in relieving pain and swelling in joints and muscles associated with osteoarthritis, rheumatoid arthritis,and gout.the drug can also be used as a short term treatment of moderate pain after dental surgery.Sku: 1713657753-2462
Etoricoxib 90 MG TAB-NUCOXIA
₦100.00 -
Etorocoxib tabs are indicated for the treatment of rheumatoid arthritis, psoriatic arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and goutSku: 1713657814-2472
Etoricoxib 90mg Atoxia Tablets 10?s
₦100.00 -
Etoricoxib Tablets, 28 TabletsEtoricoxib Tablets are the perfect solution for managing pain and inflammation. This medication contains etoricoxib as the active ingredient, a member of a group of medications known as selective COX-2 inhibitors and part of a larger family of medicines known as non-steroidal anti-inflammatory drugs (NSAIDs). Etoricoxib is proven to effectively reduce and eliminate pain, swelling, and stiffness caused by arthritis, osteoarthritis, rheumatoid arthritis, gout, ankylosing spondylitis, chronic lower back pains and other musculoskeletal conditions. In addition to its analgesic effects, Etoricoxib has also shown to reduce the occurrence of ulcers in the stomach and intestine.Etoricoxib Tablets provide long-lasting relief from chronic joint discomfort without unpleasant side effects. It is easily absorbed into the bloodstream with no need for food intake for optimal absorption. Its anti-inflammatory effect develops within 24 hours after taking it and continues throughout its duration.Etoricoxib Tablets offer trusted pain relief from various types of musculoskeletal conditions such as arthritis, gout, ankylosing spondylitis, rheumatoid arthritis and lower back pains. Talk to your doctor today about using Etoricoxib Tablets for quick relief from persistent discomfort due to these common conditions.Etoricoxib tablets are available in four strengths: 120mg, 90mg, 60mg and 30mg.Etoricoxib tablets can be used for treatment in cats and dogs when prescribed by a vetWhat is Etoricoxib used for?Etoricoxib helps to reduce the pain and swelling (inflammation) in the joints and muscles of people 16 years of age and older with osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and gout.Etoricoxib is also used for the short term treatment of moderate pain after dental surgery in people 16 years of age and older.What is osteoarthritis?Osteoarthritis is a disease of the joints. It results from the gradual breakdown of cartilage that cushions the ends of the bones. This causes swelling (inflammation), pain, tenderness, stiffness and disability.What is rheumatoid arthritis?Rheumatoid arthritis is a long term inflammatory disease of the joints. It causes pain, stiffness, swelling, and increasing loss of movement in the joints it affects. It may also cause inflammation in other areas of the body.What is gout?Gout is a disease of sudden, recurring attacks of very painful inflammation and redness in the joints. It is caused by deposits of mineral crystals in the joint.What is ankylosing spondylitis?Ankylosing spondylitis is an inflammatory disease of the spine and large jointsEtoricoxib Tablets ReviewsAfter using Etoricoxib Tablets, it’s helpful to let others know about your experience. Reviews of an item help other users know that medicines received have helped the condition it is claimed for, how well the treatment worked or any issues to be aware of. We invite our users to leave a review of both their treatment and of the service provided. Click on the reviews tab to see if there has been feedback on this item.What is the price of Etoricoxib Tablets?The price of Etoricoxib Tablets starts from £8.40Where can you buy Etoricoxib Tablets?You can buy Etoricoxib Tablets at Dock Pharmacy Essex UK, UK Online Pharmacy.Can you buy Etoricoxib Tablets Over the counter?Etoricoxib Tablets is not available to buy over the counter. You need a prescription to buy Etoricoxib TabletsSku: 1217413
Etoricoxib Tablets, 28 Tablets
₦100.00 -
etoricoxib is a non steroidal anti inflammatory drug,used in relieving pain and swelling in joints and muscles associated with osteoarthritis, rheumatoid arthritis,and gout.the drug can also be used as a short term treatment of moderate pain after dental surgery.Sku: 1713656262-2184
Etoricoxib-NUCOXIA 60MG TAB
₦100.00 -
-
-
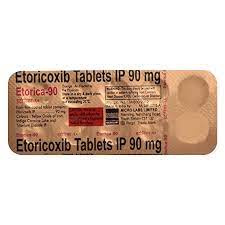
SaleSku: 1737135482-7966
ETORIL 90MG TABLETS
₦2,750.00Original price was: ₦2,750.00.₦2,268.75Current price is: ₦2,268.75.₦2,750.00Original price was: ₦2,750.00.₦2,268.75Current price is: ₦2,268.75. Add to basket Quick View