-
ROSACEA SOAP 75 GM ₦2,578.25 QTY: 1
-
Prozinc Clean Shampoo 120ml ₦655.00 QTY: 1
-
ATOREC GOLD 20MG TAB 10`S ₦1,336.50 QTY: 1
-
BLUMOX CA 625MG TAB 10`S ₦3,815.75 QTY: 1
-
First Aid Kit Small size ₦8,000.00 QTY: 1
-
Maxtin 2500Mcg Tablets 30S ₦2,137.00 QTY: 1
-
AVANAIR 200MG TAB 4`S ₦6,600.00 QTY: 1
-
Snowfire Ointment ₦9,000.00 QTY: 1
-
Spasmina Plus 30cps ₦21,356.00 QTY: 1
-
Nutrison Energy - 1000ml ₦26,200.00 QTY: 1
-
CABERLIN 0.5MG TAB 4`S ₦9,455.50 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
62257–62272 of 168331 Results
-
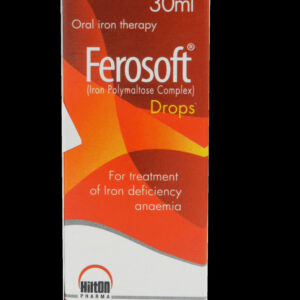
Ferosoft Drop 30ml Uses It is used to treat or prevent vitamin deficiency due to poor diet, certain illnesses, or during pregnancy. Side Effects Constipation, diarrhea, or upset stomach may occur. These effects are usually temporary and may disappear as your body adjusts to this medication. If any of these effects persist or worsen, contact your doctor or pharmacist promptly. When not to use It must not be used in the event of known hypersensitivity or intolerance to the active ingredient or one of the excipients. Precaution Before taking this product, tell your doctor or pharmacist if you are allergic to any of its ingredients; or if you have any other allergies. This product may contain inactive ingredients (such as soy found in some brands), which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Sku: 1718806805-396
Ferosoft Drop 30ml
₦11,250.00 -
Ferosoft Drops SpecificationRequires Prescription (YES/NO)YesGenericsIron (III) hydroxide polymaltose complexUsed ForAnaemiaHow it worksThe central actions of benzodiazepines are mediated through an enhancement of the GABAergic neurotransmission at inhibitory synapeses. In the presence of benzodiazepines, the affinity of the GABA receptor for the neurotransmitter is enhanced through positive allosteric modulation resulting in an increased action of released GABA on the postsynaptic transmembrance chloride ion flux.Ferosoft Drops Usage And SafetyDosageIron (III) hydroxide polymaltose complexSide EffectsOccasionally gastro-intestinal irritations such as sensation of repletion, pressure in the epigastric region, nausea, constipation or diarrhoea can occur. A dark coloration of the stool is of no clinical significance.Drug InteractionsUntil now interactions have not been observed. Since the iron is complex-bound, ionic interactions with food stuff (phenytin, oxalates, tannin, etc.) and concomitant administration of medicament (tetracycline, antacids) are unlikely to occur. IPC does not cause teeth staining. The haemoccult-test (selective for haemoglobin) for the detection of occult blood is not impaired & therefore iron therapy must not be interrupted.IndicationPrevention and treatment of all kinds of iron deficiencies particularly iron deficiency anaemiaWhen not to UseIron overload (e.g.haemochromatosis, haemosiderosis) or disturbances in iron utilization (e.g. lead anaemia, sidero achrestic, thalassaemia) and anaemias not caused by iron deficiency (e.g. haemolytic anaemia).Ferosoft Drops PrecautionsPrecautionAvoid concomitant parenteral and oral iron admin, oral iron therapy should start at least 1 wk after last iron inj.Ferosoft Drops WarningsWarning 1There is concern that a diet that is high in iron might increase the risk of heart disease in women with type 2 diabetes, although this has not been proven. If you have diabetes, discuss your iron intake with your healthcare provider.Warning 2Taking iron might cause iron overload in people with these conditions. If you have a hemoglobin disease, do not take iron unless directed by your healthcare provider.Warning 3Its Absorption is increased when taken with food.Ferosoft Drops Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716679952-1941
Ferosoft Drops 30Ml
₦23,655.00 -
-
SaleFerosoft FA Tab 20's Product Form: Tablets Pack Size: 20's Manufactured By: Hilton Pharma (Pvt) Ltd. Generic Category: Anti-Anemic Ingredients: Iron Polymaltose 100mg, Folic Acid 0.35mgSku: 1718206981-1052
Ferosoft Fa Tablet
₦259.00Original price was: ₦259.00.₦246.00Current price is: ₦246.00. -
Ferosoft Fa SpecificationRequires Prescription (YES/NO)YesGenerics?Folic Acid , Iron Hydroxide Poly Maltose Complex Used ForAnaemiaHow it worksIn order to be bound to transferrin and ferritin, iron supplements must necessarily be in a Fe+3) level of oxidation. Iron is transported to the bone marrow as ferric ion (Fe+3) bound to transferrin and is reduced to theferrous form (Fe+2) for its release in the erythroid progenitor cells and is finally transferred to the protoporphyrin for the synthesis of hemoglobin.Folic acid is required for nucleoprotein synthesis and maintenance of normal erythropoiesis; precursor of tetrahydrofolic acid, which is necessary for transformylation reactions in the biosynthesis of purines and thymidylates of nucleic acids.Ferosoft Fa Usage And SafetyDosage?Folic Acid , Iron Hydroxide Poly Maltose Complex Side EffectsThe side effects reported are occasional gastrointestinal irritation such as sensation of repletion, epigastric pain, nausea, constipation and diarrhea. There may be a dark coloration or black stools which is of no clinical significance. It does not cause teeth staining.Drug InteractionsPhenytoin , Sulfasalazine.IndicationIron (III)-hydroxide polymaltose complex + Folic acid are indicated for: - The treatment of latent iron deficiency. - Manifest iron deficiency. - Prophylactic therapy of iron and folic acid deficiency before during and after pregnancy (during lactation).When not to UseIron (III)-hydroxide polymaltose complex + Folic acid are contraindicated in patients:- Who show hypersensitivity to iron.- Receiving repeated blood transfusions.- With iron overload (e.g. hemochromatosis, hemosiderosis).- Who have disturbances in iron utilization (e.g. thalassemia, sidero-achrestic anemia, lead anemia).- Who have anemia not caused by iron deficiency (e.g. hemolytic anemia or megaloblastic anaemia caused by vitamin B12 deficiency)Ferosoft Fa PrecautionsPrecautionIn cases of anemia due to infections or malignancy, the substituted iron is stored in the reticulo-endithelial system, from which it is mobilized and utilized only after curing the primary disease.Ferosoft Fa WarningsWarning 1Since the iron is complex bound, ionic interactions with food components (phytin, oxalates, tannin etc.) and concomitant administration of drugs (tetracyclines, antacids) are unlikely to occur.Warning 2During pregnancy and lactation Iron (III)-hydroxide polymaltose complex + Folic acid should be used only if clearly required after consultation with the physician.Warning 3In case of overdosage, initially epigastric pain, diarrhea and vomiting can occur and may include metabolic acidosis, convulsions and coma after apparent recovery.Ferosoft Fa Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716679073-1818
Ferosoft Fa Tablets (1 Strip = 10 Tablets)
₦1,552.00 -
-
Ferosoft Tablets SpecificationRequires Prescription (YES/NO)YesGenericsIron (III) hydroxide polymaltose complexUsed ForAnaemiaHow it worksThe central actions of benzodiazepines are mediated through an enhancement of the GABAergic neurotransmission at inhibitory synapeses. In the presence of benzodiazepines, the affinity of the GABA receptor for the neurotransmitter is enhanced through positive allosteric modulation resulting in an increased action of released GABA on the postsynaptic transmembrance chloride ion flux.Ferosoft Tablets Usage And SafetyDosageIron (III) hydroxide polymaltose complexSide EffectsOccasionally gastro-intestinal irritations such as sensation of repletion, pressure in the epigastric region, nausea, constipation or diarrhoea can occur. A dark coloration of the stool is of no clinical significance.Drug InteractionsUntil now interactions have not been observed. Since the iron is complex-bound, ionic interactions with food stuff (phenytin, oxalates, tannin, etc.) and concomitant administration of medicament (tetracycline, antacids) are unlikely to occur. IPC does not cause teeth staining. The haemoccult-test (selective for haemoglobin) for the detection of occult blood is not impaired & therefore iron therapy must not be interrupted.IndicationPrevention and treatment of all kinds of iron deficiencies particularly iron deficiency anaemiaWhen not to UseIron overload (e.g.haemochromatosis, haemosiderosis) or disturbances in iron utilization (e.g. lead anaemia, sidero achrestic, thalassaemia) and anaemias not caused by iron deficiency (e.g. haemolytic anaemia).Ferosoft Tablets PrecautionsPrecautionAvoid concomitant parenteral and oral iron admin, oral iron therapy should start at least 1 wk after last iron inj.Ferosoft Tablets WarningsWarning 1There is concern that a diet that is high in iron might increase the risk of heart disease in women with type 2 diabetes, although this has not been proven. If you have diabetes, discuss your iron intake with your healthcare provider.Warning 2Taking iron might cause iron overload in people with these conditions. If you have a hemoglobin disease, do not take iron unless directed by your healthcare provider.Warning 3Its Absorption is increased when taken with food.Ferosoft Tablets Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716675520-1313
Ferosoft Tablets 100Mg (1 Strip = 10 Tablets)
₦1,538.00 -
Ferosoft-S Iv SpecificationRequires Prescription (YES/NO)YesGenericsFerric sucrose complexUsed ForFluids & ElectrolytesHow it worksFerric sucrose injection is in a class of medications called iron replacement products. It works?by replenishing iron stores?so that the body can make more red blood cells.Ferosoft-S Iv Usage And SafetyDosageFerric sucrose complexSide EffectsCardiac Arrthymias .Drug InteractionsAs with all parenteral iron preparations, iron sucrose should not be administered concomitantly with oral iron preparations since the absorption of oral iron is reduced. Therefore, oral iron therapy should be started at least 5 days after the last injection of Iron Sucrose.IndicationIron deficiency in cases where oral iron cannot be provided . When not to UseThe use of ferric sucrose is contra-indicated in cases of: - Known hypersensitivity to iron sucrose or any of its components. - Anemias not attributable to iron deficiency. - Iron overload or disturbances in utilization of iron. - Patients with a history of asthma, eczema or other atopic allergy, because they are more susceptible to experience allergic reactions. - Pregnancy first trimester .Ferosoft-S Iv PrecautionsPrecautionParenteral administered iron preparations can cause allergic or anaphylactoid reactions, which may be potentially fatal. Therefore, treatment for serious allergic reactions and facilities with the established cardio-pulmonary resuscitation procedures should be available.Ferosoft-S Iv WarningsWarning 1In patients with liver dysfunction, parenteral iron should only be administered after careful risk/benefit assessment. Parenteral iron administration should be avoided in patients with hepatic dysfunction where iron overload is a precipitating factor, in particular Porphyria Cutanea Tarda (PCT). Careful monitoring of iron status is recommended to avoid iron overload . Warning 2Parenteral iron must be used with caution in case of acute or chronic infection. It is recommended that the administration of iron sucrose is stopped in patients with ongoing bacteremia. In patients with chronic infection a risk/benefit evaluation has to be performed, taking into account the suppression of erythropoiesis . Warning 3Hypotensive episodes may occur if the injection is administered too rapidly. Allergic reactions, sometimes involving arthralgia, have been more commonly observed when the recommended dose is exceeded . Ferosoft-S Iv Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716665600-170
Ferosoft-S Iv Injection 100Mg (1 Box = 5 Ampoules)
₦1,881.00 -
SaleFerosom Forte Caps 20'SSku: 1727489444-1779
Ferosom Forte Caps 20’S
₦97,600.00Original price was: ₦97,600.00.₦92,232.00Current price is: ₦92,232.00.₦97,600.00Original price was: ₦97,600.00.₦92,232.00Current price is: ₦92,232.00. Add to basket Quick View -
-
-
-
Ferotein-S Iv SpecificationRequires Prescription (YES/NO)YesGenericsIron sucroseUsed ForFluids & ElectrolytesHow it worksIron sucrose injection is in a class of medications called iron replacement products. It works?by replenishing iron stores?so that the body can make more red blood cells.Ferotein-S Iv Usage And SafetyDosageIron sucroseSide EffectsCommon: transient taste perversions (in particular metallic taste). Uncommon: headache, dizziness, hypotension and collapse, tachycardia, palpitations, bronchospasm, dyspnea, nausea, vomiting, abdominal pain, diarrhea pruritus, urticaria, rash, exanthema, erythema, muscle cramps, myalgia, fever, shivering, flushing, chest pain and tightness. Injection site disorders such as superficial phlebitis, burning, swelling. Rare: paresthesia, anaphylactoid reactions (rarely involving arthralgia), peripheral edema, fatigue, asthenia, malaise. Isolated cases: reduced level of consciousness, light-headed feeling, confusion, angioedema, swelling of joints, hyperhidrosis and back pain. Hemodialyis dependent-chronic kidney disease (HDD-CKD) patients: hypotension, muscle cramps, nausea, headache, graft complications, vomiting, dizziness, hypertension, chest pain and diarrhea. Non-dialysis dependent-chronic kidney disease (NDD-CKD) patients: dysgeusia, peripheral edema, diarrhea, constipation, nausea, dizziness and hypertension . Patients receiving erythropoietin may experience: diarrhea, edema, nausea, vomiting, arthralgia, back pain, headache, hypertension, dysgeusia, dizziness, extremity pain and injection site burning. Peritoneal dialysis dependent-chronic kidney disease (PDD-CKD) patients: diarrhea, peritoneal infection, vomiting, hypertension, pharyngitis, peripheral edema and nausea.Drug InteractionsAs with all parenteral iron preparations, iron sucrose should not be administered concomitantly with oral iron preparations since the absorption of oral iron is reduced. Therefore, oral iron therapy should be started at least 5 days after the last injection of Iron Sucrose.IndicationIron sucrose is indicated for the treatment of iron deficiency in the following: - Where there is a clinical need to deliver iron rapidly to iron stores. - Patients who cannot tolerate oral iron therapy or who are noncompliant. - In active inflammatory bowel disease where oral iron preparations are ineffective. - Non-dialysis dependent-chronic kidney disease (NDD-CKD) patients receiving an erythropoietin. - Non-dialysis dependent-chronic kidney disease (NDD-CKD) patients not receiving an erythropoietin. - Hemodialysis dependent-chronic kidney disease (HDD-CKD) patients receiving an erythropoietin. - Peritoneal dialysis dependent-chronic kidney disease (HDD-CKD) patients receiving an erythropoietinWhen not to UseThe use of iron sucrose is contra-indicated in cases of: - Known hypersensitivity to iron sucrose or any of its components. - Anemias not attributable to iron deficiency. - Iron overload or disturbances in utilization of iron. - Patients with a history of asthma, eczema or other atopic allergy, because they are more susceptible to experience allergic reactions. - Pregnancy first trimester .Ferotein-S Iv PrecautionsPrecautionParenteral administered iron preparations can cause allergic or anaphylactoid reactions, which may be potentially fatal. Therefore, treatment for serious allergic reactions and facilities with the established cardio-pulmonary resuscitation procedures should be available.Ferotein-S Iv WarningsWarning 1In patients with liver dysfunction, parenteral iron should only be administered after careful risk/benefit assessment. Parenteral iron administration should be avoided in patients with hepatic dysfunction where iron overload is a precipitating factor, in particular Porphyria Cutanea Tarda (PCT). Careful monitoring of iron status is recommended to avoid iron overload . Warning 2Parenteral iron must be used with caution in case of acute or chronic infection. It is recommended that the administration of iron sucrose is stopped in patients with ongoing bacteremia. In patients with chronic infection a risk/benefit evaluation has to be performed, taking into account the suppression of erythropoiesis . Warning 3Hypotensive episodes may occur if the injection is administered too rapidly. Allergic reactions, sometimes involving arthralgia, have been more commonly observed when the recommended dose is exceeded . Ferotein-S Iv Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716664674-89
Ferotein-S Iv Injection 100Mg/5Ml (1 Box = 5 Ampoules)
₦16,625.00 -
Description For treatment and prevention of anemia. For preventing iron, folic acid acid, vitamin B12 and zinc deficiences during pregnancy, breastfeeding, after surgery or in conditions of nutritional malabsorption.Sku: 1731570697-164
FEROTONE CAPSULE BLISTER
₦1,430.00 -
FeroVance? Ferovance Red Blood Cell Boosting Nutrients 30-Day Pack helps to form red blood cells and helps in their proper function. This supplement also contains vitamin C which contributes to iron absorption from food as well as reduces tiredness and fatigue The activated form of vitamins: Do not need to be broken down prior to absorption and utilisation by the body. Iron, folate, vitamin B6 and vitamin B12: Helps to form red blood cells and helps in their proper function. Vitamin C: Contributes to iron absorption from food as well as reduces tiredness and fatigue. How to use: Take one Capsule daily with a meal, or as prescribed by your Healthcare Professional. Do not take your iron supplement with tea, coffee or a high fibre meal as this will impair iron absorption. Ingredients: Iron Amino Acid Chelate - 24 mg Folate - 400 ug Vitamin B6 - 25 mg Vitamin B12 - 25 ug Vitamin C - 250 mgSku: 1718412538-4933
FeroVance 30 Capsules
₦23,495.00