Sort by:
79857–79872 of 175597 Results
-
-
-
-
-
-
-
-
-
-
SaleSku: 1705271039-876
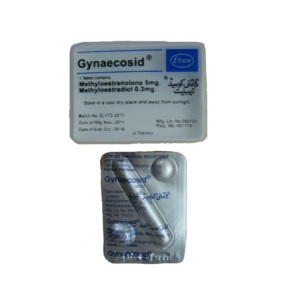
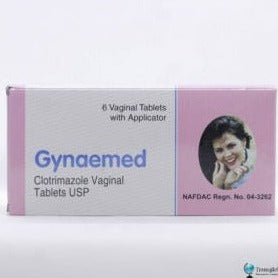
Gynaemed Cotrimazole Vaginal Tablet
₦1,200.00Original price was: ₦1,200.00.₦1,050.00Current price is: ₦1,050.00.₦1,200.00Original price was: ₦1,200.00.₦1,050.00Current price is: ₦1,050.00. Add to basket Quick View -








 No products in the cart.
No products in the cart.