-
Vichy Capital Soleil Mattifying 3-in-1 SPF50+ 50ml ₦35,545.48 QTY: 1
-
REVOSTAT 10MG STRIP OF 10 TABLETS ₦1,742.75 QTY: 1
-
CAMOSUNATE JUNIOR *6 ₦850.00 QTY: 1
-
ELAN PHARMA RINIFOL CAPSULE (10 Capsules) ₦104.00 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
102481–102496 of 163306 Results
-
Nimed Underpads-166 contains super absorbent underpads that protect bed linen, sofas, wheelchairs, and other surfaces thar may be hard to clean ? Super absorption to keep skin healthy ? Top layer made with hypoallergenic non-woven fabric ? Waterproof polyethylene back sheet to prevent leakageSku: 1715789101-118
Nimed Underpads-166
₦100.00 -
-
NIMENRIX vaccine is indicated for active immunisation for individuals from 6weeks against meningococcal disease caused by the four type; Neisseria meningitides types A, C, W and Y. This vaccine is indicated in all preteens and teens at 11 to 12 years old with a booster dose at 16 years old. Children and adults are at high risk for meningococcal disease.Sku: 1736039707-836
Nimenrix Vaccine ( Meningitis Vaccine)
₦93,600.00 -
Nimixa Tablets SpecificationRequires Prescription (YES/NO)YesGenericsRifaximinUsed ForBacterial InfectionHow it worksRifaximin acts by binding to the beta-subunit of bacterial DNA dependent RNA polymerase resulting in inhibition of bacterial RNA synthesis. Rifaximin has been shown to be active against the non invasive strain of Escherichia coli (enterotoxigenic and enteroaggregative strains). Rifaximin is believed to affect gut bacteria resulting in a decreased production and/or absorption of bacterial derived neurotoxins, including ammonia, responsible for the neurocognitive and neuromuscular dysfunction seen in patients with Hepatic Encephalopathy.Nimixa Tablets Usage And SafetyDosageRifaximinSide EffectsTraveler?s Diarrhea : Common : Flatulence, headache, abdominal pain, rectal tenesmus, defecation urgency, nausea, constipation, pyrexia, vomiting. Uncommon : Lymphocytosis, monocytosis, neutropenia, ear pain, motion sickness, tinnitus, abdominal distension, diarrhea, dry throat, fecal abnormality, gingival disorder, inguinal hernia, dry lips, stomach discomfort, chest pain, fatigue, malaise, pain, weakness, dysentery, respiratory tract infection, upper respiratory tract infection, sunburn, aspartate aminotransferase increased, blood in stool, blood in urine, weight decreased, anorexia, dehydration, arthralgia, muscle spasms, myalgia, neck pain, abnormal dreams, dizziness, migraine, syncope, loss of taste, insomnia, choluria, dysuria, hematuria, polyuria, proteinuria, urinary frequency, dyspnea, nasal passage irritation, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, rhinitis, rhinorrhea, clamminess, rash, sweating increased, hot flashes.Hepatic Encephalopathy : Common : Peripheral edema, nausea, dizziness, fatigue, ascites, muscle spasms, pruritus, abdominal pain, abdominal distension, anemia, cough, depression, insomnia, nasopharyngitis, upper abdominal pain, arthralgia, back pain, constipation, dyspnea, pyrexia, rash. Uncommon : Vertigo, lower abdominal pain, abdominal tenderness, dry mouth, esophageal variceal bleed, stomach discomfort, chest pain, generalized edema, influenza like illness, pain, cellulitis, pneumonia, rhinitis, upper respiratory tract infection, confusion, fall, procedural pain, weight increased, anorexia, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hyponatremia, myalgia, pain in extremity, amnesia, disturbance in attention, hypoesthesia, memory impairment, tremor, confusional state, epistaxis, hypotension.Drug InteractionsCYP3A4 substrates , rifamycins , substrate of P-glycoprotein.IndicationTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Rifaximin and other antibacterial drugs, Rifaximin when used to treat infection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.When not to UseRifaximin is contraindicated: - In patients with a hypersensitivity to rifaximin, any of the rifamycin antimicrobial agents, or any of the excipient of product. -During pregnancy and in women of childbearing potential not using contraception. - In -the treatment of traveler?s diarrhea caused by invas ive enteric pathogens such as Campylobacter, Salmonella and Shighella, which typically produce dysentery-like diarrhea characterized by fever , blood in the stool and high stool frequency.Nimixa Tablets PrecautionsPrecautionUpon administration of rifaximin, discontinue rifaximin if diarrhea symptoms due to pathogens other than Escherichia coli get worse or persist more than 24-48 hours and alternative antibiotic therapy should be considered.Nimixa Tablets WarningsWarning 1Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including rifaximin, and may range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.Warning 2It is not known whether rifaximin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from rifaximin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Warning 3There is increased systemic exposure in patients with severe hepatic insufficiency. Therefore, caution should be exercised when administering rifaximin to patients with severe hepatic insufficiency.Nimixa Tablets Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716676960-1501
Nimixa Tablets 200Mg (1 Box = 1 Strip)(1 Strip = 10 Tablets)
₦4,275.00 -
Nimixa Tablets SpecificationRequires Prescription (YES/NO)YesGenericsRifaximinUsed ForBacterial InfectionHow it worksRifaximin acts by binding to the beta-subunit of bacterial DNA dependent RNA polymerase resulting in inhibition of bacterial RNA synthesis. Rifaximin has been shown to be active against the non invasive strain of Escherichia coli (enterotoxigenic and enteroaggregative strains). Rifaximin is believed to affect gut bacteria resulting in a decreased production and/or absorption of bacterial derived neurotoxins, including ammonia, responsible for the neurocognitive and neuromuscular dysfunction seen in patients with Hepatic Encephalopathy.Nimixa Tablets Usage And SafetyDosageRifaximinSide EffectsTraveler?s Diarrhea : Common : Flatulence, headache, abdominal pain, rectal tenesmus, defecation urgency, nausea, constipation, pyrexia, vomiting. Uncommon : Lymphocytosis, monocytosis, neutropenia, ear pain, motion sickness, tinnitus, abdominal distension, diarrhea, dry throat, fecal abnormality, gingival disorder, inguinal hernia, dry lips, stomach discomfort, chest pain, fatigue, malaise, pain, weakness, dysentery, respiratory tract infection, upper respiratory tract infection, sunburn, aspartate aminotransferase increased, blood in stool, blood in urine, weight decreased, anorexia, dehydration, arthralgia, muscle spasms, myalgia, neck pain, abnormal dreams, dizziness, migraine, syncope, loss of taste, insomnia, choluria, dysuria, hematuria, polyuria, proteinuria, urinary frequency, dyspnea, nasal passage irritation, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, rhinitis, rhinorrhea, clamminess, rash, sweating increased, hot flashes.Hepatic Encephalopathy : Common : Peripheral edema, nausea, dizziness, fatigue, ascites, muscle spasms, pruritus, abdominal pain, abdominal distension, anemia, cough, depression, insomnia, nasopharyngitis, upper abdominal pain, arthralgia, back pain, constipation, dyspnea, pyrexia, rash. Uncommon : Vertigo, lower abdominal pain, abdominal tenderness, dry mouth, esophageal variceal bleed, stomach discomfort, chest pain, generalized edema, influenza like illness, pain, cellulitis, pneumonia, rhinitis, upper respiratory tract infection, confusion, fall, procedural pain, weight increased, anorexia, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hyponatremia, myalgia, pain in extremity, amnesia, disturbance in attention, hypoesthesia, memory impairment, tremor, confusional state, epistaxis, hypotension.Drug InteractionsCYP3A4 substrates , rifamycins , substrate of P-glycoprotein.IndicationTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Rifaximin and other antibacterial drugs, Rifaximin when used to treat infection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.When not to UseRifaximin is contraindicated: - In patients with a hypersensitivity to rifaximin, any of the rifamycin antimicrobial agents, or any of the excipient of product. -During pregnancy and in women of childbearing potential not using contraception. - In -the treatment of traveler?s diarrhea caused by invas ive enteric pathogens such as Campylobacter, Salmonella and Shighella, which typically produce dysentery-like diarrhea characterized by fever , blood in the stool and high stool frequency.Nimixa Tablets PrecautionsPrecautionUpon administration of rifaximin, discontinue rifaximin if diarrhea symptoms due to pathogens other than Escherichia coli get worse or persist more than 24-48 hours and alternative antibiotic therapy should be considered.Nimixa Tablets WarningsWarning 1Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including rifaximin, and may range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.Warning 2It is not known whether rifaximin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from rifaximin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Warning 3There is increased systemic exposure in patients with severe hepatic insufficiency. Therefore, caution should be exercised when administering rifaximin to patients with severe hepatic insufficiency.Nimixa Tablets Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716676320-1421
Nimixa Tablets 550Mg (1 Box = 1 Strip)(1 Strip = 10 Tablets)
₦9,785.00 -
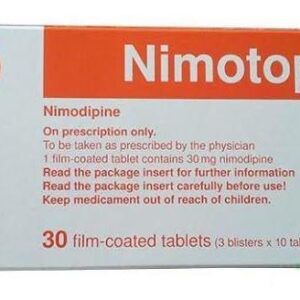
Nimoden Tablets SpecificationRequires Prescription (YES/NO)YesGenericsNimodipineUsed ForHaemorrhageHow it works?Nimodipine is a?calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells during depolarization as slow ionic transmembrane currents.Nimoden Tablets Usage And SafetyDosageNimodipineSide EffectsRash , headache , fast heartbeat , flushing, sweating, feeling of warmth , feeling sick (nausea) , constipation (lack of bowel movement) , slight rise in liver enzymes (this will show up in blood tests) , Signs of allergic reaction such as swelling of the face, lips, tongue or throat, difficulty breathing, rash, itching, nausea or vomiting , low blood pressure (may cause dizziness) , slow heartbeat , easier bruising and bleeding caused by a reduced number of blood platelets .Drug InteractionsRifampicin (an antibiotic), phenobarbital, phenytoin, or carbamazepine (anti-epileptic drugs) , including nifedipine, diltiazem, verapamil, alphamethyldopa, alpha-blockers or beta-blockers , cimetidine , sodiumvalproate , fluoxetine or nefazodone , zidovudine , indinavir, ritonavir, nelfinavir or saquinavir , ketoconazole, itraconazole or fluconazole , quinupristin / dalfopristin.IndicationIt is used to prevent changes in brain function after bleeding around the brain (subarachnoid haemorrhage).When not to UseDo not take Nimodipine: *At the same time as you are getting Nimotop solution through a drip. The tablets have been prescribed as a convenient way to continue your treatment after the drip is stopped. *If you have had a heart attack within the last month. * If you suffer from angina and notice an increase in the frequency and severity of attacks. * If you are allergic to nimodipine or any of the ingredients of this medicine . * If you are taking rifampicin (an antibiotic), phenobarbital, phenytoin or carbamazepine (three medicines most commonly used to treat epilepsy).Nimoden Tablets PrecautionsPrecautionDo not start taking Nimodipine within 4 days of drinking grapefruit juice or eating grapefruit. Tell your doctor if you have had grapefruit or grapefruit juice in this time. Also, do not drink grapefruit juice or eat grapefruit whilst taking Nimodipine . Grapefruit juice is known to increase the blood levels of the active ingredient, nimodipine. This effect can last for at least four days.Nimoden Tablets WarningsWarning 1Talk to your doctor or pharmacist before taking If you had a head injury, which caused bleeding around the brain (traumatic subarachnoid haemorrhage).Warning 2Talk to your doctor or pharmacist before taking If you have fluid in the brain or severely raised pressure in your skull. Your doctor will be able to advise you about this.Warning 3Talk to your doctor or pharmacist before taking If you have liver disease. You will probably need to have your blood pressure measured regularly.Nimoden Tablets Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716671273-763
Nimoden Tablets 30Mg (1 Strip = 10 Tablets)
₦1,774.00 -
-
Nimodipine is a drug used in the management of subarachnoid haemorrhage(bleeding into the brain)this drug is not safe in pregnant and breastfeeding mothers.Sku: 1713652511-1361
Nimodipine 30mg 30?s-NIMOTOP tablets
₦100.00 -
Nimotop 30mg Tablets 100?s belong to the class of pharmacological agents known as calcium channel blockers. The tablets improve neurological?outcome by reducing the incidence and severity of ischemic deficits in patients with?subarachnoid hemorrhage. The hemorrhage may have been cause from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition.Sku: 1719165583-1847
Nimotop 30mg Tablets 100?s
₦208,000.00 -
-
-
-
Nims?Tablets 100Mg SpecificationRequires Prescription (YES/NO)YesGenerics?NimesulideUsed ForPain & InflammationHow it worksNimesulide is a non-steroidal anti-inflammatory drug that selectively inhibits cyclooxygenase-2 but it appears but it appears to exert its therapeutic effects through variety of other mechanisms as well like inhibition of platelet activating factor ( PAF ), tumor necrosis factor-alpha (TNF-alpha ), protease & histamine etc. Nims?Tablets 100Mg Usage And SafetyDosage?NimesulideSide EffectsAt therapeutic doses nimesulide is usually well tolerated. Occasional side effects include heartburn , nausea and epigastric pain. Skin rashes of allergic type may rarely occur.Drug InteractionsAsk your physician or pharmacist for any drug interactions.IndicationPainful inflammatory conditions including those associated with osteoarthritis , postoperative trauma , sports injuries , ear , nose and throat disorders , dental surgery , bursitis/tendinitis , thrombophlebitis , pharyngitis.When not to UseThe use of nimesulide is contraindicated in patients with hepatic impairment , children , active peptic ulcer disease , known hypersensitivity to the active substances, acetylsalicylic acid , or other prostaglandin synthetase inhibiting drugs . Nims?Tablets 100Mg PrecautionsPrecautionRarely nimesulide has been reported to be associated with serious hepatic reactions , including very rare fatal cases. Patients who experience symptoms compatible with hepatic injury during treatment with nimesulide (e.g. anorexia , nausea , vomiting , abdominal pain , fatigue , dark urine ) or patients who develop abnormal liver function tests should have treatment discontinued. These patients should not be rechallenged with nimesulide. Liver damage, in mose cases reversible, has been reported following short exposure to the drug . Nims?Tablets 100Mg WarningsWarning 1Long term use of Nimesulide can cause damage to the stomach and intestine. Serious conditions like bleeding, ulceration, and perforation of the stomach or intestinal wall can occur. Any symptom indicating ulceration and bleeding like chronic indigestion, the appearance of coffee coloured dry blood in stools or vomiting of blood should be reported to your doctor immediately.Warning 2Long-term use of Nimesulide may increase the risk of a heart attack if you have pre-existing heart problems or heart failure. It is not used for treating pain after coronary bypass surgery.Warning 3Nimesulide should be used with caution in the elderly population due to an increase in the risk of undesired side effects. Close monitoring of kidney, liver and heart function and also symptoms of bleeding is necessary while receiving this medicine.Nims?Tablets 100Mg Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716681838-2218
Nims?Tablets 100Mg (1 Strip = 10 Tablets)
₦1,045.00 -
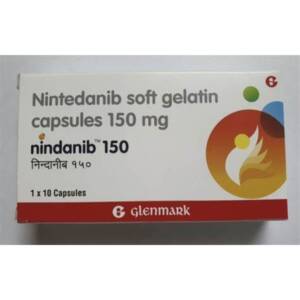
CALL TO CONFIRM AVAILABILITY AND PRICESku: 1713316900-1001
NINDANIB (NINTEDANIB) 150MG CAPSULES
₦900,500.00 -