-
Timonorm 20cpr ₦38,285.00 QTY: 5
-
PAUSE 500MG TAB 10`S ₦4,170.25 QTY: 2
-
5 Mono 10mg Tablet 10?S ₦23,800.00 QTY: 3
-
FENCETA 325MG TABLETS 15`S ₦554.75 QTY: 1
-
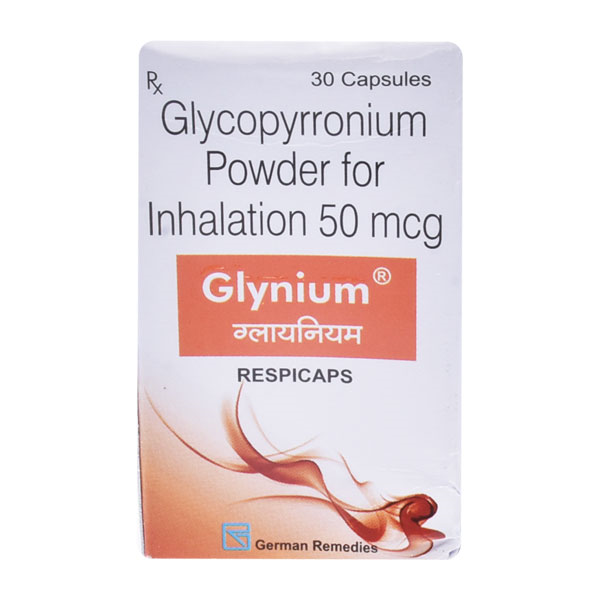
Glynium Respicap 30?s ₦49,300.00 QTY: 3
-
GLIMSER P 1MG TAB 15`S ₦3,296.00 QTY: 3
-
Beesline? Apitherapy Whitening Deodorant Roll-On Pacific Islands 50 mL ₦23,912.00 QTY: 1
-
EXIPAM 10mg Tablet 10?s ₦27,200.00 QTY: 2
-
AL KETOCER Tablet 10?s ₦30,600.00 QTY: 2
-
Goserelin Acetate 3.6mg Zoladex Injection ₦296,000.00 QTY: 3
-
Medronate 60mg Injection ₦0.00 QTY: 1
-
Quest Magnesium Citrate Tablets 250mg - 30 Pack ₦5,100.00 QTY: 1
-
AMINORICH GRANULES 5GMS POWDER ₦8,972.00 QTY: 2
-
BECTODINE 10GM POWDER ₦1,118.00 QTY: 2
-
Swedish Collagen Shots - 20/30 Pack ₦45,800.00 QTY: 1
-
Star Medical WaterJel - R2 Soothing Lotion 172 Sachets ₦101,536.00 QTY: 2
-
LILXAPLA 100mg Injection 1?s ₦270,300.00 QTY: 2
-
FOSFA Injection 1?s ₦49,300.00 QTY: 1
-
Aquasoft Cream ,100g ₦2,690.00 QTY: 1
-
COURLOL 50MG STRIP OF 10 TABLETS ₦1,197.50 QTY: 1
-
Dompi 10mg ₦0.00 QTY: 1
-
AUGUMED 625MG TAB 10`S ₦4,017.75 QTY: 2
-
Flucos ITZ Dusting Powder ₦100.00 QTY: 1
-
SPIRULINA 500MG 200CPR ₦31,673.00 QTY: 3
-
ANGICAM 5MG TAB 15`S ₦542.00 QTY: 2
-
VALPOREST CR 500 Tablet 10?s ₦27,200.00 QTY: 2
-
Machaler Device 1?s ₦40,800.00 QTY: 1
-
CILNY 20MG TAB 10`S ₦3,018.00 QTY: 2
-
CONFIDO 60`S TAB ₦3,193.00 QTY: 2
-
K18 Leave-In Molecular Repair Hair Mask ₦125,733.35 QTY: 2
-
Luprorin 3.75mg Injection 1?S ₦154,700.00 QTY: 1
-
Purina Beta Adult Dry Dog Food – Chicken ₦6,000.00 QTY: 1
-
CILAHEART T 40MG TAB 10`S ₦2,207.00 QTY: 2
-
CADIPAN 25K CAP 10`S ₦3,692.00 QTY: 2
-
TeuraLEI Menopause turaMED 60 Capsules ₦39,197.00 QTY: 2
-
Spare Bulb for the Opthalmoscope ₦100.00 QTY: 1
-
DIABETONE CAP 15`S ₦4,573.25 QTY: 3
-
ERGOFLO N Tablet 10?s ₦40,800.00 QTY: 2
-
PLACIDA PLUS TABLET ₦2,784.50 QTY: 1
-
ROSQUEL F TABLETS ₦3,062.75 QTY: 1
-
EMSTAR 1GM INJECTION ₦16,512.50 QTY: 2
-
Swanson Albion Magnesium Glycinate Powder - 150g ₦2,769.00 QTY: 1
-
CREZIO FORTE Capsule 10?s ₦88,400.00 QTY: 1
-
DERMADEW VG WASH LOTION 100ML ₦4,331.25 QTY: 1
-
ATORVA 20MG TAB 15`S ₦5,104.25 QTY: 1
-
ZavaMet 500 Tablet -10 Tab ₦7,650.00 QTY: 1
-
FORMOST 200 BOX OF 120MD METERED DOSE INHALER ₦14,540.75 QTY: 1
-
Coehb Syrup 150ml ₦37,400.00 QTY: 1
-
SIBOLONE 2.5MG STRIP OF 15 TABLETS ₦7,940.75 QTY: 1
-
DAPANORM M 5MG STRIP OF 10 TABLETS ₦1,712.00 QTY: 1
-
AANIJ 1.8 ml Storage Vials, ASFSY0359 (Pack of 25) ₦100.00 QTY: 1
-
MASON VITAMIN C 500MG ₦4,750.00 QTY: 1
-
FLAVATE 200MG TABLET ₦5,012.00 QTY: 1
Customer matched zone "Lagos Delivery Options"
“FLAVATE 200MG TABLET” has been added to your basket. Continue shopping
Sort by:
104641–104656 of 174026 Results
-
Nalidixic acid is a prescription drug given orally for the treatment of urinary tract infections caused by susceptible bacteria. Avoid use of this medicine if you have a history of kidney failure and or epilepsy.Sku: 1713653407-1567
Nalidixic Acid 500mg Agonal Tablet 10?s
₦100.00 -
Nalidixic acid is a prescription drug given orally for the treatment of urinary tract infections caused by susceptible bacteria. Avoid use of this medicine if you have a history of kidney failure and or epilepsySku: 1713649428-640
Nalidixic Acid 500mg Uriseptic Tablet 10?s
₦100.00 -
SaleNaloc Fungal Nail Treatment - 10mlSku: 1716302670-1594
Naloc Fungal Nail Treatment – 10ml
₦44,900.00Original price was: ₦44,900.00.₦29,900.00Current price is: ₦29,900.00.₦44,900.00Original price was: ₦44,900.00.₦29,900.00Current price is: ₦29,900.00. Add to basket Quick View -
-
Therapeutic indications For use as an additional therapy within a comprehensive treatment program including psychological guidance for detoxified patients who have been opioid-dependent & alcohol dependence to support abstinence. Posology and method of administration Use in adults Naltrexone treatment should be initiated and supervised by suitable qualified physicians. The initial dose of naltrexone hydrochloride should be 25 mg (half a tablet) for opioid-dependent patient followed by the usual dose of one tablet per day (= 50 mg naltrexone hydrochloride) A missed dose can be managed by providing 1 tablet per day each day till the next regular dosage-administration. Naltrexone administered to opioid-dependent persons can cause life-threatening withdrawal symptoms. Patients suspected of using or being addicted to opioids must undergo a naloxone provocation test , unless it can be verified that the patient has not taken any opioids for 7-10 days (urine test) prior to the initiation of treatment with naltrexone. As Naltrexone is an adjunctive therapy and the full recovery process in opioid-dependent patients is individually variable, no standard duration of treatment can be stated; an initial period of three months should be considered. However, prolonged administration may be necessary. The recommended dose for alcohol dependence to support abstinence is 50 mg per day (1 tablet). A dose of over 150 mg on any single day is not recommended, since this can lead to a higher incidence of side effects. As naltrexone hydrochloride is an adjunctive therapy and the full recovery process from alcohol dependence is individually variable, no standard duration of treatment can be stated; an initial period of three months should be considered. However, prolonged administration may be necessary The dosage-regimen can be modified in order to improve compliance to a three-times-a-week dosing schedule as follows: administration of 2 tablets (=100 mg naltrexone hydrochloride) on Monday and on Wednesday and 3 tablets (=150 mg naltrexone hydrochloride) on Friday. Lactose Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine. Interaction with other medicinal products and other forms of interaction Concomitant administration of naltrexone with an opioid-containing medication should be avoided. Fertility, pregnancy and lactation Pregancy There are no clinical data on naltrexone hydrochloride use in pregnancy. Data from animal studies have shown reproductive toxicity. The data are insufficient to establish clinical relevance. The potential risk for humans is unknown. Naltrexone should only be given to pregnant women when, in the judgement of the attending physician the potential benefits outweigh and the possible risk. The use of naltrexone in pregnant alcoholic patients receiving long-term treatment with opiates or substitution treatment with opiates, or in pregnant patients who are opioid-dependent, creates a risk of acute withdrawal syndrome which could have serious consequences for the mother and the foetus. Naltrexone administration must be suspended if opiate analgesics are prescribed. Lactation: There are no clinical data on naltrexone HCl use in lactation. It is unknown whether naltrexone or 6-beta-naltrexol is excreted in human breast milk. During treatment breast feeding is not recommended. Adverse effects Very common (At least 1 in every 10 people) Nervousness Anxiety Insomnia Headache Sleep disorders Restlessness Abdominal pain Abdominal cramps Nausea or inclination to vomit Vomitting Arthralgia- Joint stiffness. Myalgia-Muscle pain Feebleness Asthenia-The body loses strength Common(?1/100 to <1/10) Decreased appetite Affective disorders Despondency- A period of low spirit Irritability Mood swings Dizziness Shivering Vertigo Lycrimation increase Tachycardia Palpitations Electrocardiogram change Chest pain Diarrhoea Constipation Skin rash Urine retention Delayed ejaculation Lack of appetite Thirst Increased energy Chills Uncommon (?1/1,000 to < 1/100) Oral herpes Tinea pedis- Athletes foot Lymphadenopathy Hallucinations Confusion state Depression Paranoia Disorientation Nightmares Agitation Libido disorder Abnormal dreams Rare (? 1/10,000 to < 1/1,000) Idiopathic thrombocytopenia Suicidal Ideation Attempted suicide Speech disorder Very rare (< 1/10,000) Euphoria Exanthema-Diffuse skin rash Rhabdomyolysis- The breakdown of muscle tissue that leads to the release of muscle fiber contents into the blood.Sku: 1714345285-625
NALOREX NALTREXONE 50MG TABS 28S
₦100.00 -
-
-
-
Naloxone HCl API ? High Purity, Pharmaceutical GradeSku: 1750187171-51
Naloxone HCl API ? High Purity, Pharmaceutical Grade
₦0.00 -
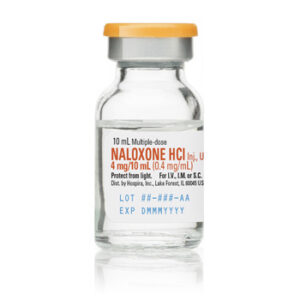
Multiple Dose Glass Fliptop Vial, 1 x 25 x 10mL Naloxone Hydrochloride Injection, USP Brand Name Equivalent: Narcan NDC 00409-1219-01 / 00409121901 Concentration: 0.4 mg/mL Content: 0.4 mg/10mL Non-DEHP Preservative-Free Non-ReturnableSku: 1718317854-832
Naloxone Hydrochloride Injection, USP Brand Name Equivalent: Narcan 0.4mg/10mL Multiple Dose Glass Fliptop Vial 1 x 25 x 10mL
₦100.00 -
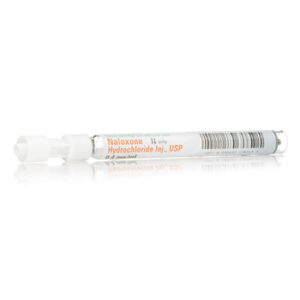
Carpuject? Luer Lock Glass Syringe (no needle).? 1 mL fill in 2.5 mL Carpuject? Single-dose cartridge with Luer Lock for the Carpuject? Syringe System 1 x 10 x 1mL Naloxone Hydrochloride Injection, USP Brand Name Equivalent: Narcan NDC 00409-1782-69 / 00409178269 Concentration: 0.4 Mm/mL Content: 0.4 mg/mL Non-DEHP Preservative-Free All Carpuject? Luer Lock Glass Syringe (no needle) products require the use of the Carpuject? holder, list number 02049-02, for administration. Non-ReturnableSku: 1718317851-831
Naloxone Hydrochloride Injection, USP Brand Name Equivalent: Narcan Carpuject Luer Lock Glass Syringe (no needle) 1 x 10 x 1mL
₦100.00 -
NALOXONE hydrochloride, 0.4mg/ml, 1ml, amp.Sku: 1723588682-500
NALOXONE hydrochloride, 0.4mg/ml, 1ml, amp.
₦0.00 -
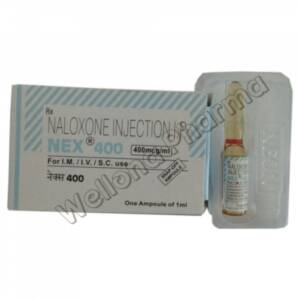
Naloxone Injection Trade Name: Nex Available Strength : 400 mcg Packing : Ampoules Pack Insert/Leaflet : Yes Therapeutic use : Antipsychotic,Generic Drugs Production Capacity : 1 million injection/monthSku: 1714922726-194
Naloxone Injection
₦100.00 -
-