-
NuroKor KorGlov Device Accessory ₦0.00 QTY: 1
-
Cetaphil Sun Light Gel SPF50 100ml ₦97,112.00 QTY: 1
-
Ultra-White Soap 75gm ₦424.00 QTY: 1
-
Vichy Liftactiv Supreme Vitamin C Serum 20ml ₦79,278.82 QTY: 1
Customer matched zone "Lagos Delivery Options"
Sort by:
85025–85040 of 379872 Results
-
SaleD-Sefa 500 mgNigella Sativa (Black Seed Oil) is indicated for the treatment of common cold, cough, asthma, bronchitis, muscle spasm, fever, dyspepsia, vomiting, gout, insufficient breast milk, eczema and wound. It is also used to improve immune system, reduce blood sugar, blood pressure & stop hair loss.Theropeutic ClassHerbal and NutraceuticalsPharmacologyIts purpose is to support the immune and respiratory functions, such as- in the treatment of asthma, whooping cough and respiratory disorders. Nigellon also has anti-histamine properties as compared to thymoquinone.Dosage & Administration of D-Sefa 500 mg1 capsule 2-3 times daily or as directed by the registered physicianInteraction of D-Sefa 500 mgNigella Sativa (Black Seed Oil) can be taken with any other vitamins, minerals or herbal supplement.ContraindicationsThere is no known contraindication.Side Effects of D-Sefa 500 mgNo side effects have been reportedPregnancy & LactationBlack seed oil is not recommended during pregnancy. In lactating mother it should be taken to increase breast milk of mother.Precautions & WarningsThere is no known precautionStorage ConditionsKeep away from direct sunlight and moisture, keep below 30?C temperature in cool and dry place. Keep the medicine out of reach of children.Sku: 1736103924-3407
D-Sefa500 mg
₦440.00Original price was: ₦440.00.₦396.00Current price is: ₦396.00. -
-
-
D-Soft Softgel SpecificationRequires Prescription (YES/NO)YesGenericsCholecalciferol Used ForVitamin D SupplementHow it worksCholecalciferol (vitamin D3) is formed in the skin under influence of UV rays and metabolized in two hydroxylation steps at first in the liver and then in the kidney tissue into the biological active metabolite 1,25-dihydroxy-cholecalciferol. 1,25-dihydroxy-cholecalciferol is involved fundamentally in the regulation of the calcium and phosphate balance together with parathyroid hormone and calcitonin.D-Soft Softgel Usage And SafetyDosageCholecalciferol Side EffectsAcute symptoms : Arrhythmias, nausea, vomiting, psychic symptoms, and impaired consciousnesses.Chronic symptoms : Polyuria, polydipsia, weight loss, kidney stone formation, nephrocalcinosis, extraosseous calcificationsDrug InteractionsThiazide diuretics , Phenytoin or barbiturates , Glucocorticoids , Digoxin , Antacids (Magnesium-containing) , Cholestyramine, Colestipol, Mineral Oil.Indication1. As dietary supplement for prevention of Vitamin D deficiency in adults and children.2. Prevention of Vitamin D deficiency in high risk patients with malabsorption, certain chronic diseases, disorders of metabolism,drugs or other causes.3. Treatment of vitamin D deficiency states like rickets, osteomalacia and osteoporosis.In all cases calcium supplements must be provided along with Vitamin D supplementationWhen not to UseIt may not be used in the following conditions:Hypersensitivity to Cholecalciferol or any of the ingredients of the drug.Hypercalcemia and/or hypercalciuria.It should not be administered to patients : With a tendency towards the formation of kidney stones containing calcium ; With pseudohypoparathyroidism.D-Soft Softgel PrecautionsPrecautionIf other vitamin D containing drugs are administered, the dosage of Cholecalciferol must be taken into account. Additional administration of vitamin D or calcium should be carried out only under medical monitoring. In such cases the calcium levels in the serum and urine must be supervised.D-Soft Softgel WarningsWarning 1It should be administered only with caution to patients with a disturbed renal calcium and phosphate excretion.Warning 2In the case of hypercalcemia or reduced kidney function the dosage must be reduced or the therapy discontinued.Warning 3It should be administered only with caution to patients suffering from sarcoidosis since the risk of transformation of vitamin D into its active metabolites is increased.D-Soft Softgel Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716681820-2215
D-Soft Softgel 1000Iu (1 Bottle = 30 Softgels)
₦6,175.00 -
-
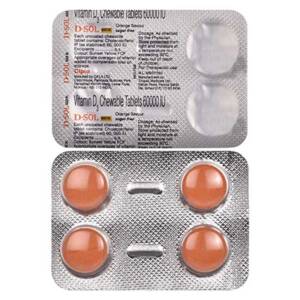
D-Sol 60K Vitamin D3 Chewable Tablet Sugar Free Orange 4'sSku: 1713480487-188
D-Sol 60K Vitamin D3 Chewable Tablet Sugar Free Orange 4’s
₦100.00 -
Product OverviewD-Sorbitol Powder for molecular biology, 98%9910350-70-4Molecular Formula : C6H14O6 Molecular Weight: 182.18 Part A Storage: Room Temperature Shelf Life: 60 Months HSN Code: 29054400 IMDG Identification: Not Regulated for Transport (Non-Haz)Package Size: 500 GProduct More SpecificationsMade InIndiaThere are no reviews for this product.Write a review Your Name Your ReviewNote: HTML is not translated! Rating Bad Good Captcha Please complete the captcha validation below ContinueSku: 1721367370-7906
D-Sorbitol Powder for molecular biology, 98% ,500 G
₦100.00 -
SaleD-STONE TABLET (30 TAblets)Sku: 1728570654-1720
D-STONE TABLET (30 TAblets)
₦2,850.00Original price was: ₦2,850.00.₦2,700.00Current price is: ₦2,700.00.₦2,850.00Original price was: ₦2,850.00.₦2,700.00Current price is: ₦2,700.00. Add to basket Quick View -
Rohs_Mouser: YSku: 78607965
D-Sub Standard Connectors DSUB B25P RA318, (Pack of 20) (571-5788794-1)
₦106,837.75 -
-
-
SaleD-Toin 100 mgParenteral?Phenytoin is indicated for the treatment of generalized tonic-clonic?status epilepticus, and prevention and treatment of seizures occurring during neurosurgery. Intravenous Phenytoin can also be substituted, as short-term use, for oral?phenytoin. Parenteral Phenytoin should be used only when oral Phenytoin administration is not possibleTheropeutic ClassAdjunct anti-epileptic drugsPharmacologyPhenytoin acts as an anticonvulsant by increasing efflux or decreasing influx of sodium ions across cell membranes in the motor cortex during generation of nerve impulses; thus stabilising neuronal membranes and decreasing seizure activity. It acts as an antiarrhythmic by extending the effective refractory period and suppressing ventricular pacemaker automaticity, shortening action potential in the heart.Dosage of D-Toin 100 mgOral:?Epilepsy: Adult: Initially, 3-4 mg/kg daily as single dose or in divided doses. Alternatively, 150-300 mg daily increased gradually to 600 mg daily if necessary. Maintenance: 200-500 mg daily. Child: Initially, 5 mg/kg daily in 2-3 divided doses. Maintenance: 4-8 mg/kg daily in divided doses. Max dose: 300 mg daily. Intravenous:?Tonic-clonic status epilepticus: Adult: Adjunctive therapy with a benzodiazepine (e.g. diazepam): 10-15 mg/kg by slow inj or intermittent infusion at a max rate of 50 mg/min. Maintenance: 100 mg IV (or orally) given every 6-8 hr. Child: Neonates: 20 mg/kg as a loading dose, then 2.5-5 mg/kg bid; 1 mth-12 yr: 18 mg/kg as a loading dose, then 2.5-5 mg/kg bid; >12 yr: 18 mg/kg as a loading dose, then up to 100 mg 3-4 times daily. Administration of D-Toin 100 mgShould be taken with food. When administering to patients on nasogastric or other enteral feeds, do not administer feeds 2 hr before or after a dose. Be consistent throughout therapy in relation to feed times. Do not switch dosage forms/brands w/o prior consideration.Interaction of D-Toin 100 mgEffects with other sedative drugs or ethanol may be potentiated. Enhances toxic effects of paracetamol, lithium. Increased risk of osteomalacia with acetazolamide. Decreased serum levels/effects with acyclovir, antineoplastics, benzodiazeines, ciprofloxacin, CYP2C9 inducers (e.g. carbamazepine), CYP2C19 inducers (e.g. rifampin), folic acid, vigabatrin. Increased serum concentrations with allopurinol, capecitabine, cimetidine, CYP2C9 inhibitors (e.g. fluconazole), CYP2C19 inhibitors (e.g. delavirdine), disulfiram, methylphenidate, metronidazole, omeprazole, SSRI, trazodone, trimethoprim. Increases metabolism of antiarrhythmics, anticonvulsants, antipsychotics, beta-blockers, calcium channel blockers, chloramphenicol, corticosteroids, doxycycline, oestrogens, HMG-CoA reductase inhibitors, methadone, theophylline, TCAs. Decreases levels/effects of clozapine, ciclosporin, tacrolimus, CYP2B6 substrates (e.g. bupropion, selegiline), CYP2C8 substrates (e.g. amiodarone), CYP2C9 substrates (e.g. celecoxib), CYP2C19 substrates (e.g. citalopram), CYP3A4 substrates (e.g. benzodiazepines), digoxin, itraconazole, levodopa, neuromuscular-blocking agents, thyroid hormones, topiramate. Increases levels/effect of dopamine, ticlopidine. Valproic acid may displace phenytoin from binding sites; and affect phenytoin serum concentrations. Transiently increases the hypothrombinaemia response to warfarin initially, followed by an inhibition of the response.ContraindicationsD-Toin 100 mg is contraindicated in patients with: A history of hypersensitivity to phenytoin, its inactive ingredients, or other hydantoins? Sinus bradycardia, sino-atrial?block, second and third degree A-V block, and Adams-Stokes syndrome because of the effect of?parenteral?phenytoin on?ventricular?automaticity. A history of prior acute hepatotoxicity attributable to phenytoin? Coadministration with delavirdine because of the potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside?reverse transcriptase?inhibitors. Side Effects of D-Toin 100 mgHypersensitivity, lack of appetite, headache, dizziness, tremor, transient nervousness, insomnia, GI disturbances (e.g. nausea, vomiting, constipation), tenderness and hyperplasia of the gums, acne, hirsutism, coarsening of the facial features, rashes, osteomalacia. Phenytoin toxicity as manifested as a syndrome of cerebellar, vestibular, ocular effects, notably nystagmus, diplopia, slurred speech, and ataxia; also with mental confusion, dyskinesias, exacerbations of seizure frequency, hyperglycaemia. Solutions for inj may cause local irritation or phlebitis. Prolonged use may produce subtle effects on mental function and cognition, especially in children.Pregnancy & LactationCategory D: There is positive evidence of human foetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).Precautions & WarningsCardiovascular disease, e.g. sinus bradycardia, heart blocks; DM; hepatic impairment; hypoalbuminemia; porphyria; seizures (may increase frequency of petit mal seizures); debilitated patients; elderly. Caution in IV admin in hypotension, heart failure or MI, monitor BP and ECG during therapy. IV must be given slowly (too rapid admin may cause hypotension, CNS depression, cardiac arrhythmias and impaired heart conduction). Extravasation and intra-arterial admin must be avoided. Do not discontinue abruptly (may increase seizure frequency), unless safety concerns require a more rapid withdrawal. May impair ability to drive or operate machinery.Overdose Effects of D-Toin 100 mgThe?lethal?dose in pediatric patients is not known. The lethal dose in adults is estimated to be 2 to 5 grams. The initial symptoms are?nystagmus,?ataxia, and?dysarthria. Other signs are?tremor, hyperreflexia,?lethargy, slurred speech, blurred vision, nausea, and vomiting. The patient may become comatose and?hypotensive. Death is caused by respiratory and?circulatory?depression.There are marked variations among individuals with respect to?phenytoin?serum levels where toxicity may occur. Nystagmus, on?lateral?gaze, usually appears at 20 mcg/mL, ataxia at 30 mcg/mL, dysarthria and lethargy appear when the serum concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery. Irreversible cerebellar dysfunction and?atrophy?have been reported.Treatment: Treatment is nonspecific since there is no known?antidote. The adequacy of the respiratory and circulatory systems should be carefully observed and appropriate supportive measures employed.?Hemodialysis?can be considered since phenytoin is not completely bound to plasma proteins. Total exchange?transfusion?has been used in the treatment of severe intoxication in pediatric patients. In acute overdosage the possibility of other CNS depressants, including alcohol, should be borne in mind.Storage ConditionsIntravenous: Store at room temperature of 15-30?C.Oral:? Tablet/capsule: Store below 30?C. Protect from light and moisture; Oral suspension: Store at room temperature of 20-25?C, do not freeze, protect from light. Use In Special PopulationsPediatric Use: A loading dose of 15 to 20 mg/kg of Phenytoin intravenously will usually produce serum concentrations of phenytoin within the generally accepted serum total concentrations between 10 and 20 mcg/mL (unbound phenytoin concentrations of 1 to 2 mcg/mL). Because of the increased risk of adverse cardiovascular reactions associated with rapid administration Phenytoin should be injected slowly intravenously at a rate not exceeding 1 to 3 mg/kg/min or 50 mg per minute, whichever is slowerGeriatric Use: Phenytoin clearance tends to decrease with increasing age. Lower or less frequent dosing may be required?Renal and Hepatic Impairment Or Hypoalbuminemia: The liver is the site of biotransformation. Patients with impaired liver function, elderly patients, or those who are gravely ill may show early toxicity. Because the fraction of unbound phenytoin is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients.Drug ClassesAdjunct anti-epileptic drugsMode Of ActionPhenytoin acts as an anticonvulsant by increasing efflux or decreasing influx of sodium ions across cell membranes in the motor cortex during generation of nerve impulses; thus stabilising neuronal membranes and decreasing seizure activity. It acts as an antiarrhythmic by extending the effective refractory period and suppressing ventricular pacemaker automaticity, shortening action potential in the heart.PregnancyCategory D: There is positive evidence of human foetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).Pediatric UsesPediatric Use: A loading dose of 15 to 20 mg/kg of Phenytoin intravenously will usually produce serum concentrations of phenytoin within the generally accepted serum total concentrations between 10 and 20 mcg/mL (unbound phenytoin concentrations of 1 to 2 mcg/mL). Because of the increased risk of adverse cardiovascular reactions associated with rapid administration Phenytoin should be injected slowly intravenously at a rate not exceeding 1 to 3 mg/kg/min or 50 mg per minute, whichever is slowerGeriatric Use: Phenytoin clearance tends to decrease with increasing age. Lower or less frequent dosing may be required?Renal and Hepatic Impairment Or Hypoalbuminemia: The liver is the site of biotransformation. Patients with impaired liver function, elderly patients, or those who are gravely ill may show early toxicity. Because the fraction of unbound phenytoin is increased in patients with renal or hepatic disease, or in those with hypoalbuminemia, the monitoring of phenytoin serum levels should be based on the unbound fraction in those patients.Sku: 1736106263-4094
D-Toin100 mg
₦2,200.00Original price was: ₦2,200.00.₦1,980.00Current price is: ₦1,980.00.₦2,200.00Original price was: ₦2,200.00.₦1,980.00Current price is: ₦1,980.00. Add to basket Quick View -
D-Tres 200,000Iu SpecificationRequires Prescription (YES/NO)YesGenericsCholecalciferol Used ForVitamin D SupplementHow it worksCholecalciferol (vitamin D3) is formed in the skin under influence of UV rays and metabolized in two hydroxylation steps at first in the liver and then in the kidney tissue into the biological active metabolite 1,25-dihydroxy-cholecalciferol. 1,25-dihydroxy-cholecalciferol is involved fundamentally in the regulation of the calcium and phosphate balance together with parathyroid hormone and calcitonin.D-Tres 200,000Iu Usage And SafetyDosageCholecalciferol Side EffectsAcute symptoms : Arrhythmias, nausea, vomiting, psychic symptoms, and impaired consciousnesses.Chronic symptoms : Polyuria, polydipsia, weight loss, kidney stone formation, nephrocalcinosis, extraosseous calcificationsDrug InteractionsThiazide diuretics , Phenytoin or barbiturates , glucocorticoidsIndicationPrevention of Vitamin D Deficiency due to malabsorption, drugs, certain chronic diseases, disorders of metabolism or other causes. Treatment of vitamin D deficiency states. These include the following: Rickets: In children , Osteomalacia: In adults.When not to UseIt may not be used in the following conditions:Hypersensitivity to Cholecalciferol or any of the ingredients of the drug.Hypercalcemia and/or hypercalciuria.It should not be administered to patients : With a tendency towards the formation of kidney stones containing calcium ; With pseudohypoparathyroidism.D-Tres 200,000Iu PrecautionsPrecautionIf other vitamin D containing drugs are administered, the dosage of Cholecalciferol must be taken into account. Additional administration of vitamin D or calcium should be carried out only under medical monitoring. In such cases the calcium levels in the serum and urine must be supervised.D-Tres 200,000Iu WarningsWarning 1It should be administered only with caution to patients with a disturbed renal calcium and phosphate excretion.Warning 2In the case of hypercalcemia or reduced kidney function the dosage must be reduced or the therapy discontinued.Warning 3Dan-D should be administered only with caution to patients suffering from sarcoidosis since the risk of transformation of vitamin D into its active metabolites is increased.D-Tres 200,000Iu Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716676818-1485
D-Tres 200,000Iu Oral/Im Injection 5Mg/Ml (1 Box = 1 Injection)
₦18,065.00 -
D-Tres Oral/Im SpecificationRequires Prescription (YES/NO)YesGenericsCholecalciferol Used ForVitamin D SupplementHow it worksCholecalciferol (vitamin D3) is formed in the skin under influence of UV rays and metabolized in two hydroxylation steps at first in the liver and then in the kidney tissue into the biological active metabolite 1,25-dihydroxy-cholecalciferol. 1,25-dihydroxy-cholecalciferol is involved fundamentally in the regulation of the calcium and phosphate balance together with parathyroid hormone and calcitonin.D-Tres Oral/Im Usage And SafetyDosageCholecalciferol Side EffectsAcute symptoms : Arrhythmias, nausea, vomiting, psychic symptoms, and impaired consciousnesses.Chronic symptoms : Polyuria, polydipsia, weight loss, kidney stone formation, nephrocalcinosis, extraosseous calcificationsDrug InteractionsThiazide diuretics , Phenytoin or barbiturates , glucocorticoidsIndicationPrevention of Vitamin D Deficiency due to malabsorption, drugs, certain chronic diseases, disorders of metabolism or other causes. Treatment of vitamin D deficiency states. These include the following: Rickets: In children , Osteomalacia: In adults.When not to UseIt may not be used in the following conditions:Hypersensitivity to Cholecalciferol or any of the ingredients of the drug.Hypercalcemia and/or hypercalciuria.It should not be administered to patients : With a tendency towards the formation of kidney stones containing calcium ; With pseudohypoparathyroidism.D-Tres Oral/Im PrecautionsPrecautionIf other vitamin D containing drugs are administered, the dosage of Cholecalciferol must be taken into account. Additional administration of vitamin D or calcium should be carried out only under medical monitoring. In such cases the calcium levels in the serum and urine must be supervised.D-Tres Oral/Im WarningsWarning 1It should be administered only with caution to patients with a disturbed renal calcium and phosphate excretion.Warning 2In the case of hypercalcemia or reduced kidney function the dosage must be reduced or the therapy discontinued.Warning 3Dan-D should be administered only with caution to patients suffering from sarcoidosis since the risk of transformation of vitamin D into its active metabolites is increased.D-Tres Oral/Im Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716675126-1260
D-Tres Oral/Im Injection 5Mg (1 Box = 5 Injection)
₦855.00 -
D-Va Drops SpecificationRequires Prescription (YES/NO)YesGenericsVitamin D3Used ForVitamin D SupplementHow it worksCholecalciferol (vitamin D3) is formed in the skin under influence of UV rays and metabolized in two hydroxylation steps at first in the liver and then in the kidney tissue into the biological active metabolite 1,25-dihydroxy-cholecalciferol. 1,25-dihydroxy-cholecalciferol is involved fundamentally in the regulation of the calcium and phosphate balance together with parathyroid hormone and calcitonin.D-Va Drops Usage And SafetyDosageVitamin D3Side EffectsAcute symptoms : Arrhythmias, nausea, vomiting, psychic symptoms, and impaired consciousnesses.Chronic symptoms : Polyuria, polydipsia, weight loss, kidney stone formation, nephrocalcinosis, extraosseous calcificationsDrug InteractionsThiazide diuretics , Phenytoin or barbiturates , Glucocorticoids , Digoxin , Antacids (Magnesium-containing) , Cholestyramine, Colestipol, Mineral Oil.Indication1. As dietary supplement for prevention of Vitamin D deficiency in adults and children.2. Prevention of Vitamin D deficiency in high risk patients with malabsorption, certain chronic diseases, disorders of metabolism, drugs or other causes.3. Treatment of vitamin D deficiency states like rickets, osteomalacia and osteoporosis.In all cases calcium supplements must be provided along with Vitamin D supplementationWhen not to UseIt may not be used in the following conditions:Hypersensitivity to Cholecalciferol or any of the ingredients of the drug.Hypercalcemia and/or hypercalciuria.It should not be administered to patients : With a tendency towards the formation of kidney stones containing calcium ; With pseudohypoparathyroidism.D-Va Drops PrecautionsPrecautionIf other vitamin D containing drugs are administered, the dosage of Cholecalciferol must be taken into account. Additional administration of vitamin D or calcium should be carried out only under medical monitoring. In such cases the calcium levels in the serum and urine must be supervised.D-Va Drops WarningsWarning 1It should be administered only with caution to patients with a disturbed renal calcium and phosphate excretion.Warning 2In the case of hypercalcemia or reduced kidney function the dosage must be reduced or the therapy discontinued.Warning 3It should be administered only with caution to patients suffering from sarcoidosis since the risk of transformation of vitamin D into its active metabolites is increased.D-Va Drops Additional InformationPregnancy categoryAlways consult your physician before using any medicine.Storage (YES/NO)Store this medicine at room temperature, away from direct light and heat.Sku: 1716667800-380
D-Va Drops 10Ml
₦39,188.00 -