
The First 20 Elements
Elements may be identified using their atomic number, element name, or element symbol. The symbol is a one- or two-letter abbreviation of the name. However, some symbols refer to old element names. For example, the symbol for sodium is Na. This refers to the Latin word natrium, which was the old name for caustic soda. The symbol of potassium is K, which stands for the Latin word kalium, which meant alkali or potash.
The first letter of an element symbol is capitalized. When there is a second letter, it is lower case.
A Closer Look at the First 20 Elements
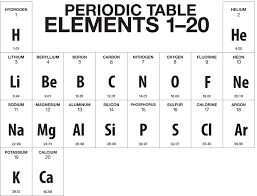
Here is a brief description of each of the first 20 elements, including appearance, state of matter under normal temperature and pressure, atomic number, symbol, atomic mass, electron configuration, and element group:
Hydrogen
- Hydrogen is a nonmetallic, colorless gas under ordinary conditions. Under extreme pressure, it becomes an alkali metal. There are three isotopes of this element, which differ in the number of neutrons in their atoms. The most common isotope is protium. The others are deuterium and tritium.
- Atomic Number: 1
- Symbol: H
- Atomic Mass: 1.008
- Electron Configuration: 1s1
- Group: group 1, s-block, nonmetal
Helium
- Helium is a light, colorless gas.
- Atomic Number: 2
- Symbol: He
- Atomic Mass: 4.002602(2)
- Electron Configuration: 1s2
- Group: group 18, s-block, noble gas
Lithium
Lithium is a highly reactive metal.
- Lithium is a reactive silver solid metal.
- Atomic Number: 3
- Symbol: Li
- Atomic Mass: 6.94 (6.938–6.997)
- Electron Configuration: [He] 2s1
- Group: group 1, s-block, alkali metal
Beryllium
- Beryllium is a shiny gray-white solid metal.
- Atomic Number: 4
- Symbol: Be
- Atomic Mass: 9.0121831(5)
- Electron Configuration: [He] 2s2
- Group: group 2, s-block, alkaline earth metal
Boron
- Boron is a gray solid with a metallic luster.
- Atomic Number: 5
- Symbol: B
- Atomic Mass: 10.81 (10.806–10.821)
- Electron Configuration: [He] 2s2 2p1
- Group: group 13, p-block, metalloid
Carbon
- Carbon is a solid that takes several forms, include diamond, graphite, and amorphous carbon. It is black, gray, or colorless.
- Atomic Number: 6
- Symbol: C
- Atomic Mass: 12.011 (12.0096–12.0116)
- Electron Configuration: [He] 2s2 2p2
- Group: group 14, p-block, usually a nonmetal although sometimes considered a metalloid
Nitrogen
- Nitrogen is a colorless gas.
- Atomic Number: 7
- Symbol: N
- Atomic Mass: 14.007
- Electron Configuration: [He] 2s2 2p3
- Group: group 15 (pnictogens), p-block, nonmetal
Oxygen
- Oxygen is a colorless gas. Its liquid form is blue, while its solid takes many colors, including red, metallic, and black.
- Atomic Number: 8
- Symbol: O
- Atomic Mass: 15.999 or 16.00
- Electron Configuration: [He] 2s2 2p4
- Group: group 16 (chalcogens), p-block, nonmetal
Fluorine
- Fluorine is a pale yellow gas and liquid and bright yellow solid.
- Atomic Number: 9
- Symbol: F
- Atomic Mass: 18.998403163(6)
- Electron Configuration: [He] 2s2 2p5
- Group: group 17, p-block, halogen
Neon
- Neon is a colorless gas that emits an orange-red glow when excited in an electric field.
- Atomic Number: 10
- Symbol: Ne
- Atomic Mass: 20.1797(6)
- Electron Configuration: [He] 2s2 2p6
- Group: group 18, p-block, noble gas
Sodium
- Sodium is a soft, silvery-white solid metal.
- Atomic Number: 11
- Symbol: Na
- Atomic Mass: 22.98976928(2)
- Electron Configuration: [Ne] 3s1
- Group: group 1, s-block, alkali metal
Magnesium
- Magnesium is a shiny gray solid metal.
- Atomic Number: 12
- Symbol: Mg
- Atomic Mass: 24.305
- Electron Configuration: [Ne] 3s2
- Group: group 2, s-block, alkaline earth metal
Aluminum
- Aluminum is a soft, silver-colored, nonmagnetic metal.
- Atomic Number: 13
- Symbol: Al
- Atomic Mass: 26.9815385(7)
- Electron Configuration: [Ne] 3s2 3p1
- Group: group 13, p-block, considered a post-transition metal or sometimes a metalloid
Silicon
- Silicon is a hard, blue-gray crystalline solid that has a metallic luster.
- Atomic Number: 14
- Symbol: Si
- Atomic Mass: 28.085
- Electron Configuration: [Ne] 3s2 3p2
- Group: group 14 (carbon group), p-block, metalloid
Phosphorus
- Phosphorus is a solid under ordinary conditions, but it takes several forms. The most common are white phosphorus and red phosphorus.
- Atomic Number: 15
- Symbol: P
- Atomic Mass: 30.973761998(5)
- Electron Configuration: [Ne] 3s2 3p3
- Group: group 15 (pnictogens), p-block, usually considered a nonmetal, but sometimes a metalloid
Sulfur
Sulfur is a yellow nonmetal.
- Sulfur is a yellow solid, usually found as a crystal or powder.
- Atomic Number: 16
- Symbol: S
- Atomic Mass: 32.06
- Electron Configuration: [Ne] 3s2 3p4
- Group: group 16 (chalcogens), p-block, nonmetal
Chlorine
- Chlorine is a pale yellow-green gas under ordinary conditions. Its liquid form is bright yellow.
- Atomic Number: 17
- Symbol: Cl
- Atomic Mass: 35.45
- Electron Configuration: [Ne] 3s2 3p5
- Group: group 17, p-block, halogen
Argon
- Argon is a colorless gas, liquid, and solid. It is a gas under ordinary conditions. It emits a bright lilac-purple glow when excited in an electric field.
- Atomic Number: 18
- Symbol: Ar
- Atomic Mass: 39.948(1)
- Electron Configuration: [Ne] 3s2 3p6
- Group: group 18, p-block, noble gas
Potassium
- Potassium is a reactive, silvery solid metal.
- Atomic Number: 19
- Symbol: K
- Atomic Mass: 39.0983(1)
- Electron Configuration: [Ar] 4s1
- Group: group 1, s-block, alkali metal
Calcium
- Calcium is a dull silver solid metal with a faint yellowish cast.
- Atomic Number: 20
- Symbol: Ca
- Atomic Mass: 40.078(4)
- Electron Configuration: [Ar] 4s2
- Group: group 2, s-block, alkaline earth metal
Here is the list of The First 20 Elements, please leave your comment below
References & Credits:



















